Physical Equilibrium
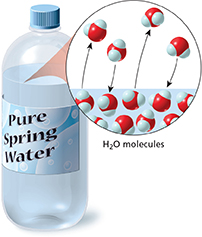
What happens when you pour some water into a jar and then close the lid? You might think that nothing happens at all. But in fact, some of the water undergoes a physical change by evaporating. As more water evaporates, some of the water vapor condenses. Eventually, the rate of evaporation equals the rate of condensation, and the system reaches equilibrium as shown in Figure 25.
When liquid water is in equilibrium with water vapor, you can describe the system by writing this equation.
Here, l stands for liquid and g stands for gas. The pair of arrows in this equation indicates that the forward change (evaporation) and the reverse change (condensation) are happening simultaneously and at the same rate. Both the forward and reverse changes are physical changes, so this equation represents a physical equilibrium.  When a physical change does not go to completion, a physical equilibrium is established between the forward and reverse changes.
When a physical change does not go to completion, a physical equilibrium is established between the forward and reverse changes.
Chemical Equilibrium All the chemical equations you have seen so far have been written with single arrows, which suggest that all reactions go to completion in one direction. In reality, however, most reactions are reversible to some extent. A reversible reaction is a reaction in which the conversion of reactants into products and the conversion of products into reactants can happen simultaneously.
In the previous section, you read about the synthesis of sulfur trioxide from sulfur dioxide and oxygen. This is actually a reversible reaction that can be expressed as
If sulfur dioxide and oxygen are mixed in a closed container, the forward reaction will start to produce sulfur trioxide. However, once molecules of sulfur trioxide form, some of them will change back into the reactants by the reverse reaction. Eventually, the rate of the forward reaction (synthesis) will equal the rate of the reverse reaction (decomposition), and the system will reach equilibrium.  When a chemical reaction does not go to completion, a chemical equilibrium is established between the forward and reverse reactions. During chemical equilibrium, the reactants change into products just as fast as the products change back into reactants.
When a chemical reaction does not go to completion, a chemical equilibrium is established between the forward and reverse reactions. During chemical equilibrium, the reactants change into products just as fast as the products change back into reactants.
Figure 25 Liquid water left in a closed container eventually reaches equilibrium with its vapor. Interpreting Diagrams What do the arrows represent in the diagram above?

What happens during chemical equilibrium?





