| Types of Solutions | ||
|---|---|---|
| Solute | Solvent | Example |
| Gas | Gas | Air (oxygen, carbon dioxide in nitrogen) |
| Liquid | Gas | Water in air |
| Gas | Liquid | Carbonated beverage (carbon dioxide in water) |
| Liquid | Liquid | Vinegar (acetic acid in water) |
| Solid | Liquid | Sugar water (sugar in water) |
| Solid | Solid | Stainless steel (Chromium and nickel in iron) |
Dissolving
Recall that a solution is a homogeneous mixture of two or more substances. Every solution has two types of components. A solute is a substance whose particles are dissolved in a solution. The substance in which the solute dissolves is called the solvent. For example, seawater is a solution in which salt is the solute and water is the solvent.
Solutes and solvents can take the form of a solid, liquid, or gas. The solution takes the state of the solvent. Figure 2 lists some common solutions and the states of their respective solutes and solvents. Air, for instance, is a solution of several gases dissolved in another gas. Nitrogen, making up about 78 percent of air, is the solvent. Oxygen, carbon dioxide, argon, and other gases are solutes.
Figure 2 A stainless steel pot or pan is a solution of chromium and nickel in iron. In a solution, the solvent is the substance in the greatest quantity.
 dd
ddYou are probably most familiar with solutions in which water is the solvent. Carbonated drinks, hot tea, and seawater are just a few examples of the many water-based solutions you might have encoun-tered.  Substances can dissolve in water in three ways—by dissociation, dispersion, and ionization.
Substances can dissolve in water in three ways—by dissociation, dispersion, and ionization.
Dissociation of Ionic Compounds
For a solute to dissolve in water, the solute and solvent particles must attract one another. However, the particles within the solute are attracted to one another, and the particles within the solvent are attracted to one another. So before a solution can form, the attractions that hold the solute together and the solvent together must be overcome.
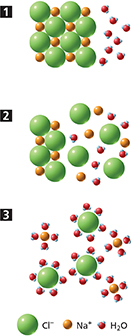
Figure 3 illustrates how a sodium chloride crystal dissolves in water. Sodium chloride is an ionic compound. Water is a polar molecule, and is attracted to the ions in the solute. The crystal dissolves as the sodium and chlorine ions are pulled into solution, one by one, by the surrounding water molecules. The process in which an ionic compound separates into ions as it dissolves is called dissociation.
Figure 3 When an ionic compound dissolves in water, the charged ends of water molecules surround the oppositely charged ions.
 d
d




 How does sodium chloride dissolve in water?
How does sodium chloride dissolve in water?