3.3 Phase Changes
Reading Focus
Key Concepts
 What are six common phase changes?
What are six common phase changes? What happens to a substance's temperature and a system's energy during a phase change?
What happens to a substance's temperature and a system's energy during a phase change? How does the arrangement of water molecules change during melting and freezing?
How does the arrangement of water molecules change during melting and freezing? How are evaporation and boiling different?
How are evaporation and boiling different?
Vocabulary
phase change
endothermic
heat of fusion
exothermic
vaporization
heat of vaporization
evaporation
vapor pressure
condensation
sublimation
deposition
Reading Strategy
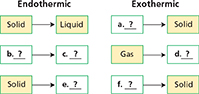
Summarizing Copy the diagram. As you read, complete the description of energy flow during phase changes.
Massive chunks of frozen water called icebergs are a common sight off the continent of Antarctica. A large iceberg like the one in Figure 15 contains enough fresh water to supply millions of people with water for a year. During the summer in southern Australia, fresh water is a scarce resource. People have proposed towing icebergs to Australia from Antarctica. The plan has not been implemented because the trip could take months to complete and much of the iceberg would melt along the way. In this section, you will find out what happens when a substance, such as water, changes from one state to another.
Figure 15 The solid and liquid phases of water are visible in this photograph of an iceberg in the Amundsen Sea near Antarctica.

Characteristics of Phase Changes
When at least two states of the same substance are present, scientists describe each different state as a phase. For example, if an iceberg is floating in the ocean, there are two phases of water present—a solid phase and a liquid phase. A phase change is the reversible physical change that occurs when a substance changes from one state of matter to another.




