Chemical Calculations
Think about baking a cake like the one in Figure 7. The directions on a box of cake mix might tell you to add two eggs and one cup of water to the cake mix. Suppose you wanted to make three cakes. Although the directions don't tell you specifically how many eggs and how much water are required for three cakes, you could figure out the amounts. To make three cakes, you would need three times as much of each ingredient—six eggs, three cups of water, and three packages of cake mix.
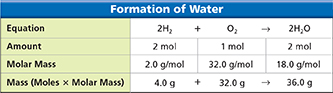
Chemical equations can be read as recipes for making new substances. Figure 8 shows the balanced equation for the formation of water. You can read this equation as, “Two molecules of hydrogen react with one molecule of oxygen and form two molecules of water.” In terms of moles, the equation reads, “Two moles of hydrogen react with one mole of oxygen and form two moles of water.” To convert from moles to mass, you need to use the molar masses as conversion factors. Figure 8 shows how the same equation can also be read as, “4.0 grams of H2 reacts with 32.0 grams of O2 and forms 36.0 grams of H2O.”
How many grams of oxygen would you need to make 144 grams of water? In chemical reactions, the mass of a reactant or product can be calculated by using a balanced chemical equation and molar masses of the reactants and products. The chemical equation tells you how to relate amounts of reactants to amounts of products. Molar masses let you convert those amounts into masses.
Figure 7 A cake recipe tells you how much of each ingredient to use for each cake you bake.
Using Analogies How is a cake recipe like a chemical equation?


How do you convert from moles to mass?
Converting Mass to Moles
To calculate how much oxygen is required to make 144 grams of water, you need to begin with a balanced chemical equation for the reaction.
The first step in your calculations is to determine how many moles of water you are trying to make. By using the molar mass of water, you can convert the given mass of water into moles.
Figure 8 In a balanced chemical equation, the number of atoms of each element on the left equals the number of atoms of each element on the right. By using molar masses, you can show that the mass of the reactants equals the mass of the products.
 d
d