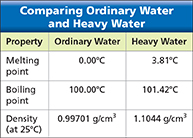
Figure 12 Heavy water contains hydrogen-2 atoms, which have twice the mass of hydrogen-1 atoms. Using Tables At what temperature would a sample of heavy water freeze?
 d
dIsotopes
In Dalton's atomic theory, all the atoms of a given element are identical. Every atom of a given element does have the same number of protons and electrons. But every atom of a given element does not have the same number of neutrons.  Isotopes are atoms of the same element that have different numbers of neutrons and different mass numbers. Isotopes of an element have the same atomic number but different mass numbers because they have different numbers of neutrons.
Isotopes are atoms of the same element that have different numbers of neutrons and different mass numbers. Isotopes of an element have the same atomic number but different mass numbers because they have different numbers of neutrons.
For example, every atom of oxygen has 8 protons. Some oxygen atoms have 8 neutrons and a mass number of 16. Some oxygen atoms have 9 neutrons and a mass number of 17. Some oxygen atoms have 10 neutrons and a mass number of 18. When it is important to distinguish one oxygen isotope from another, the isotopes are referred to as oxygen-16, oxygen-17, and oxygen-18. All three oxygen isotopes can react with hydrogen to form water or combine with iron to form rust.
With most elements, it is hard to notice any differences in the physical or chemical properties of their isotopes. Hydrogen is an exception. Hydrogen-1 has no neutrons. (Almost all hydrogen is hydrogen-1.) Hydrogen-2 has one neutron, and hydrogen-3 has two neutrons. Because a hydrogen-1 atom has only one proton, adding a neutron doubles its mass. Water that contains hydrogen-2 atoms in place of hydrogen-1 atoms is called heavy water. Figure 12 compares some physical properties of ordinary water and heavy water.
Section 4.2 Assessment
Reviewing Concepts
 Name three subatomic particles.
Name three subatomic particles. Name three properties you could use to distinguish a proton from an electron.
Name three properties you could use to distinguish a proton from an electron. Which characteristic of an atom always varies among atoms of different elements?
Which characteristic of an atom always varies among atoms of different elements? How are the isotopes of an element different from one another?
How are the isotopes of an element different from one another?What do neutrons and protons have in common? How are they different?
How can atoms be neutral if they contain charged particles?
What is the difference between atoms of oxygen-16 and oxygen-17?
Critical Thinking
Comparing and Contrasting What property do protons and electrons have that neutrons do not?
Applying Concepts Explain why it isn't possible for an atom to have a mass number of 10 and an atomic number of 12.
Connecting Concepts
Elements In Section 2.1, you were told that elements contain only one type of atom. How would you define “type of atom” to account for the existence of isotopes?




