CHAPTER 4 Assessment
Reviewing Content
Choose the letter that best answers the question or completes the statement.
One of the first people to state that matter is made up of atoms was
Democritus.
Aristotle.
Dalton.
Rutherford.
Dalton's model of an atom is best described as
a solar system.
a solid sphere.
a plum pudding.
an electron cloud.
Who provided the first evidence that atoms contain subatomic particles?
Dalton
Rutherford
Thomson
Bohr
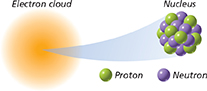
Almost all the mass of an atom is located in its
protons.
electrons.
electron cloud.
nucleus.
An electron is a particle with
a negative charge, found in the nucleus.
a positive charge, found in the nucleus.
no charge, found outside the nucleus.
a negative charge, found outside the nucleus.
Which particle is the least massive?
proton
electron
neutron
nucleus
All atoms of an element have the same
mass number.
number of isotopes.
atomic number.
number of neutrons.
The number of neutrons in an atom equals the
mass number minus atomic number.
atomic number plus number of electrons.
mass number plus atomic number.
atomic number minus mass number.
The atomic number of sulfur is 16. How many electrons are there in an atom of sulfur-34?
16
34
18
50
Atoms emit energy as light when
electrons move to a higher energy level.
electrons move to a lower energy level.
protons move to a higher energy level.
protons move to a lower energy level.
Understanding Concepts
Why must indirect evidence be used to study the structure of atoms?
What evidence convinced Dalton that elements must be made of individual particles called atoms?
In Thomson's experiment, why was the glowing beam repelled by a negatively charged plate?
What evidence supported Thomson's hypothesis that the negative particles he observed came from inside atoms?
Compare the mass and volume of the nucleus to the total mass and volume of an atom.
Compare the relative masses of protons, neutrons, and electrons in an atom.
What is the difference between the atomic number of an atom and its mass number?
If the atomic number of an atom is 11, how many electrons does the atom have? Explain.
If an atom has an atomic number of 6 and a mass number of 14, how many protons, electrons, and neutrons are in the atom?
What part of Dalton's theory was modified after the discovery of isotopes?
Which isotope of oxygen is represented by the drawing—oxygen-16, oxygen-17, or oxygen-18? Assume that all the protons and neutrons in the nucleus are visible in the drawing. Give a reason for your answer.
What is the main difference between Bohr's model of the atom and the atomic theory that is currently accepted?
What does it mean to say that an atom is in an excited state?





