Critical Thinking
Controlling Variables Look at the drawing of the experimental setup in Figure 5A. Explain how the setup is a control for the setup in Figure 5B.
Predicting How would the results of Thomson's experiment change if the beam were a stream of neutrons instead of a stream of electrons?
Interpreting Diagrams The atomic number of carbon is 6. The atomic number of nitrogen is 7. The atomic number of oxygen is 8. Name the isotope represented by the drawing.
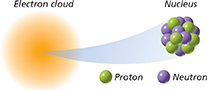
Hypothesizing Why were the proton and electron discovered before the neutron?
Applying Concepts Explain why a neutral atom cannot have one proton, one neutron, and two electrons.
Classifying The nucleus of an atom contains six neutrons and six protons. The nucleus of a second atom contains six neutrons and five protons. Are they atoms of different elements or isotopes of the same element? Explain your answer.
Math Skills
Calculating The atomic number for iron is 26. How many neutrons are in the nucleus of an iron atom with a mass number of 57? How many electrons does the iron atom have?
Applying Concepts If a potassium atom has an atomic number of 19 and a mass number of 39, how many protons, electrons, and neutrons are in the atom?
Applying Concepts A helium-4 atom has twice as many protons as a hydrogen atom. How many protons and how many neutrons are in the nucleus of a helium-4 atom?
Concepts in Action
Comparing and Contrasting The compound in blood that carries oxygen to cells throughout the body contains iron. Iron has an atomic number of 26. Iron-59 is used to diagnose disorders in the blood. How is iron-59 different from all other isotopes of iron? How is it the same?
Using Analogies Scientists working in the field of nanotechnology use either a top-down or bottom-up approach to construct tiny objects. Give an example of a visible structure that was made using the bottom-up approach and one that was made using the top-down approach.
Inferring If you see a green color when fireworks explode, can you be certain that the fireworks contained a barium compound? Give a reason for your answer.
Relating Cause and Effect Brightly colored neon lights consist of tubes filled with a gas. When an electric current passes through the tubes, different colors are emitted. Why might you conclude that the tubes in a multicolored display contain more than one element?
Writing in Science Better technology leads to an increase in scientific knowledge. An increase in knowledge allows for the invention of new technology. Write a paragraph discussing these statements. Use a scanning tunneling microscope as your example.
Performance-Based Assessment
Preparing a Survey Write ten questions you could ask to find out what people know about the modern model of an atom. Figure out the best order for the questions to test someone's knowledge fairly. Be prepared to explain your choices.





