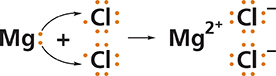Ionization Energy
An electron can move to a higher energy level when an atom absorbs energy. Cations form when electrons gain enough energy to escape from atoms. The energy allows electrons to overcome the attraction of the protons in the nucleus. The amount of energy used to remove an electron is called ionization energy. It varies from element to element. The lower the ionization energy, the easier it is to remove an electron from an atom.
Figure 3 Ionization energies generally increase from left to right across a period.
Interpreting Diagrams What is the trend for ionization energy within a group?
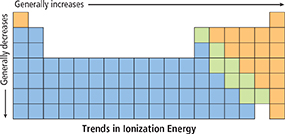
Figure 3 shows two trends for ionization energy. Ionization energies tend to increase from left to right across a period. It takes more energy to remove an electron from a nonmetal than from a metal in the same period. Ionization energies tend to decrease from the top of a group to the bottom. In Group 1A, potassium has a lower ionization energy than sodium. So it is easier to remove an electron from potassium than from sodium, and potassium is more reactive than sodium.

What is ionization energy?
Ionic Compounds
Compounds that contain ionic bonds are ionic compounds, which can be represented by chemical formulas. A chemical formula is a notation that shows what elements a compound contains and the ratio of the atoms or ions of these elements in the compound. The chemical formula for sodium chloride is NaCl. From the formula, you can tell that there is one sodium ion for each chloride ion in sodium chloride.
Based on the diagram in Figure 4, what would the formula for magnesium chloride be? A magnesium atom cannot reach a stable electron configuration by reacting with just one chlorine atom. It must transfer electrons to two chlorine atoms. After the transfer, the charge on the magnesium ion is 21 and its symbol is Mg2+1. The formula for the compound is MgCl2. The 2 written to the right and slightly below the symbol for chlorine is a subscript. Subscripts are used to show the relative numbers of atoms of the elements present. If there is only one atom of an element in the formula, no subscript is needed.
Figure 4 Magnesium chloride forms when magnesium atoms transfer electrons to chlorine atoms. Magnesium chloride is used to control dust that is stirred up by traffic on unpaved roads.