Unsaturated Hydrocarbons
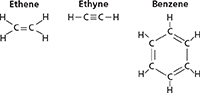
A hydrocarbon that contains one or more double or triple bonds is an unsaturated hydrocarbon. These hydrocarbons are classified by bond type and the arrangement of their carbon atoms.  There are three types of unsaturated hydrocarbons—alkenes, alkynes, and aromatic hydrocarbons.
There are three types of unsaturated hydrocarbons—alkenes, alkynes, and aromatic hydrocarbons.
Alkenes
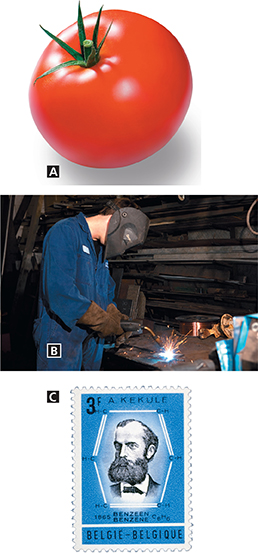
Many fruit-bearing plants produce ethene (C2H4), which controls the rate at which fruits, such as the tomato in Figure 6A, ripen. There is a double bond between the two carbon atoms in ethene. Hydrocarbons that have one or more carbon-carbon double bonds are alkenes. The names of alkenes end in −ene. Plastics used in trash bags and milk jugs are produced by reactions involving ethene.
Figure 6 There are three types of unsaturated hydrocarbons. A Ethene is an alkene that controls the ripening of a tomato. B Ethyne is an alkyne used in torches that cut metals or weld them together. C Kekulé figured out the ring structure of the aromatic hydrocarbon benzene.
Alkynes
In 1895, Henry-Louis Le Châtelier reported that a flame produced when ethyne burned had a temperature about 1000°C higher than a flame produced when hydrogen burns. Ethyne (C2H2), also known as acetylene, is an alkyne. Alkynes are straight- or branched-chain hydrocarbons that have one or more triple bonds. Alkyne names end in −yne.
Alkynes are the most reactive hydrocarbon compounds. The welder in Figure 6B is burning a mixture of oxygen and acetylene in an oxyacetylene torch. The temperature of the flame produced approaches 3500°C. At that temperature, most metals can be melted and welded together. The acetylene and oxygen are stored under pressure in tanks.
Aromatic Hydrocarbons
The Belgian stamp in Figure 6C honors Friedrich Kekulé (1829−1896), who figured out that benzene (C6H6) is an unsaturated hydrocarbon with a ring structure. Although the formula shows alternating single and double bonds, the six bonds in the ring are identical. Six of the valence electrons are shared by all six carbon atoms. Hydrocarbons that contain similar ring structures are known as aromatic hydrocarbons. The name was chosen because many of these compounds have strong aromas or odors.

What is an unsaturated hydrocarbon?




