Figure 18 illustrates the fission of a uranium-235 nucleus. Notice that one of the products of the reaction is energy.  In nuclear fission, tremendous amounts of energy can be produced from very small amounts of mass. For example, the nuclear energy released by the fission of 1 kilogram of uranium-235 is equivalent to the chemical energy produced by burning more than 17,000 kilograms of coal.
In nuclear fission, tremendous amounts of energy can be produced from very small amounts of mass. For example, the nuclear energy released by the fission of 1 kilogram of uranium-235 is equivalent to the chemical energy produced by burning more than 17,000 kilograms of coal.
Figure 18 The fission of uranium-235 yields smaller nuclei, neutrons, and energy. The nuclear equation for this reaction can be written as follows.
Comparing and Contrasting How does fission differ from nuclear decay?
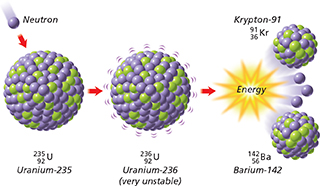
Converting Mass Into Energy In the nuclear equation shown in Figure 18, the mass numbers on the left equal the mass numbers on the right. Yet when the fission of uranium-235 is carried out, about 0.1 percent of the mass of the reactants is lost during the reaction. This “lost” mass is converted into energy.
In 1905, more than 30 years before the discovery of fission, physicist Albert Einstein had introduced the mass-energy equation. It describes how mass and energy are related.
Mass-Energy Equation
In the mass-energy equation, E represents energy, m represents mass, and c represents the speed of light . The conversion of a small amount of mass releases an enormous amount of energy. Likewise, a large amount of energy can be converted into a small amount of mass. The explosion of the first atomic bomb in 1945 offered a powerful demonstration of the mass-energy equation. The bomb contained 5 kilograms of plutonium-239. Fission of the plutonium produced an explosion that was equivalent to 18,600 tons of TNT.
Recall how the law of conservation of mass applied to chemical reactions. In nuclear reactions, however, the energies involved are much larger. To account for the conversion of mass into energy, a modified conservation law is used. According to the law of conservation of mass and energy, the total amount of mass and energy remains constant.





