Classes of Elements
The periodic table in Figure 7 presents three different ways to classify elements. First, elements are classified as solids, liquids, or gases, based on their states at room temperature. The symbols for solids are black. The symbols for liquids are purple. The symbols for gases are red.
Second, elements are divided into those that occur naturally and those that do not. All but two elements with atomic numbers 1 through 92 occur on Earth. Elements with atomic numbers of 93 and higher do not occur naturally. The symbols for these elements are white. In Chapter 10, you will find out how elements that do not occur in nature are produced.
The third classification system puts elements into categories based on their general properties.  Elements are classified as metals, nonmetals, and metalloids. In the periodic table, metals are located on the left, nonmetals are on the right, and metalloids are in between.
Elements are classified as metals, nonmetals, and metalloids. In the periodic table, metals are located on the left, nonmetals are on the right, and metalloids are in between.
Metals
The majority of the elements on the periodic table are classified as metals. In Figure 7, they are represented by blue boxes. Metals are elements that are good conductors of electric current and heat. Except for mercury, metals are solids at room temperature. Most metals are malleable. Many metals are ductile; that is, they can be drawn into thin wires.
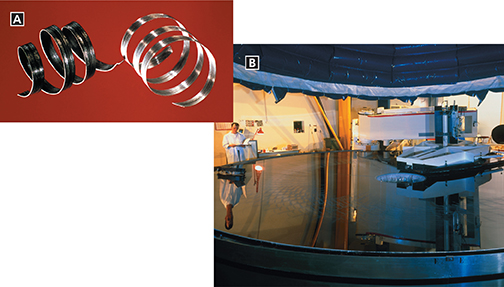
Some metals are extremely reactive and some do not react easily. One way to demonstrate this difference is to compare the behavior of gold and the behavior of magnesium when these metals are exposed to the oxygen in air. Gold remains shiny because it does not react with the oxygen. Magnesium reacts with the oxygen and quickly dulls. Figure 10A shows one magnesium coil that is dull and one that is shiny. Figure 10B shows one use for a metal with a shiny surface.
Quick Lab
Defining a Metal
Procedure 
Use forceps to put a piece of magnesium into a test tube in a test tube rack. Using a graduated cylinder, add5 mL of hydrochloric acid to the test tube.
CAUTION Wear plastic gloves because the acid can burn skin or clothing. Record your observations.
Repeat Step 1 with sulfur, aluminum, and silicon.
Analyze and Conclude
Classifying Based on their locations in the periodic table, classify the four elements as metals, metalloids, or nonmetals.
Comparing and Contrasting Compare the behavior of the metals with the acid to the behavior of the other elements with the acid.
Forming Operational Definitions Use your observations to write a definition of a metal.
Figure 10 Magnesium and aluminum are typical metals. A When magnesium reacts with oxygen, a dull layer forms on its surface. The layer can be removed to reveal magnesium's shiny surface. B Many telescope mirrors are coated with aluminum to produce a surface that reflects light extremely well.