CHAPTER 7 Study Guide
7.1 Describing Reactions
 Key Concepts
Key Concepts
The law of conservation of mass states that mass is neither created nor destroyed.
In order to show that mass is conserved during a reaction, a chemical equation must be balanced.
Because chemical reactions often involve large numbers of small particles, chemists use a unit called the mole to measure amounts of a substance.
In chemical reactions, the mass of a reactant or product can be calculated by using a balanced chemical equation and molar masses.
Vocabulary
reactants, p. 192; products, p. 192; chemical equation, p. 193; coefficients, p. 194; mole, p. 195; molar mass, p. 196
7.2 Types of Reactions
 Key Concepts
Key Concepts
The general types of chemical reactions are synthesis reactions, decomposition reactions, single-replacement reactions, double-replacement reactions, and combustion reactions.
Scientists classify certain chemical reactions as transfers of electrons between atoms.
Vocabulary
synthesis reaction, p. 200; decomposition reaction, p. 200; single-replacement reaction, p. 202; double-replacement reaction, p. 203; combustion reaction, p. 204; oxidation-reduction reaction, p. 204
7.3 Energy Changes in Reactions
 Key Concepts
Key Concepts
Chemical reactions involve the breaking of are described bycan be classified as chemical bonds in the reactants and the formation of chemical bonds in the products.
During a chemical reaction, energy is either released or absorbed.
Vocabulary
chemical energy, p. 206; exothermic reaction, p. 208; endothermic reaction, p. 209
7.4 Reaction Rates
 Key Concepts
Key Concepts
Reaction rates tell you how fast a reaction is going.
Factors that affect reaction rates include temperature, surface area, concentration, stirring, and catalysts.
Vocabulary
reaction rate, p. 212; catalyst, p. 215
7.5 Equilibrium
 Key Concepts
Key Concepts
When a physical change does not go to completion, a physical equilibrium is established between the forward and reverse changes. When a chemical reaction does not go to completion, a chemical equilibrium is established between the forward and reverse reactions.
When a change is introduced to a system in equilibrium, the equilibrium shifts in the direction that relieves the change.
Vocabulary
equilibrium, p. 216; reversible reaction, p. 217
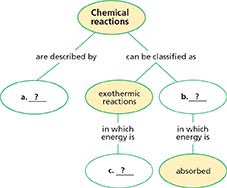
Thinking Visually
Concept Map Use information from the chapter to complete the concept map below.