To describe the burning of charcoal, you can substitute the reactants and products of the reaction into the word equation as follows.

You can then simplify the word equation by writing the reactants and products as chemical formulas.
Now you have a chemical equation. A chemical equation is a representation of a chemical reaction in which the reactants and products are expressed as formulas. You can read the equation above as, “Carbon and oxygen react and form carbon dioxide,” or, “The reaction of carbon and oxygen yields carbon dioxide.”
What is a chemical equation?
Conservation of Mass
As a piece of charcoal burns, it gets smaller and smaller until it is finally reduced to a tiny pile of ash. Although the charcoal seems to disappear as it burns, it is actually being converted into carbon dioxide gas. If you measured the mass of the carbon dioxide produced, it would equal the mass of the charcoal and oxygen that reacted.
During chemical reactions, the mass of the products is always equal to the mass of the reactants. This principle, established by French chemist Antoine Lavoisier (1743–1794), is known as the law of conservation of mass.  The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction. Recall that mass is a measure of the amount of matter. So, this law is also known as the law of conservation of matter. By demonstrating that mass is conserved in various reactions, Lavoisier laid the foundation for modern chemistry.
The law of conservation of mass states that mass is neither created nor destroyed in a chemical reaction. Recall that mass is a measure of the amount of matter. So, this law is also known as the law of conservation of matter. By demonstrating that mass is conserved in various reactions, Lavoisier laid the foundation for modern chemistry.
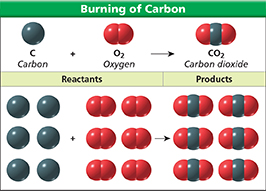
Figure 2 Whether you burn one carbon atom or six carbon atoms, the equation used to describe the reaction is the same.
Using Models How do both models of the reaction below show that mass is conserved?
 d
dFigure 2 illustrates how a chemical equation can be restated in terms of atoms and molecules. The equation reads, “One atom of carbon reacts with one molecule of oxygen and forms one molecule of carbon dioxide.” Suppose you have six carbon atoms. If each carbon atom reacts with one oxygen molecule to form one carbon dioxide molecule, then six carbon atoms react with six oxygen molecules to form six carbon dioxide molecules. Notice that the number of atoms on the left side of the equation equals the number of atoms on the right. The equation shows that mass is conserved.






