Quick Lab
Analyzing Inks
Materials
test paper, metric ruler, felt-tip markers, stapler, beaker, alcohol-water mixture, Petri dish
Procedure 
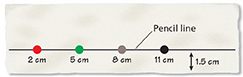
Place the test paper on a clean surface. Use the ruler to draw the pencil line shown in the drawing. Use your markers to place color dots at the locations shown in the drawing.
With the ink marks on the outside, staple the two ends of the paper together to form a tube.
Pour the alcohol-water mixture into the beaker to a depth of 0.5 cm. Stand the paper in the beaker so that the dots are at the bottom. The paper should not touch the sides of the beaker. Invert the Petri dish over the beaker.
When the mixture reaches the top of the paper, remove the paper from the beaker. Unstaple the paper and lay it flat. Make a drawing of the results with each colored area labeled.
Analyze and Conclude
Observing Which markers contained inks that were mixtures of colored substances?
Formulating Hypotheses How did some molecules in the ink move up the paper?
Predicting Assume that molecules in the test paper are more polar than molecules in the alcohol-water mixture. Would you expect the most polar molecules in ink to stick tightly to the paper or to move with the liquid? Explain.
Designing Experiments How could the procedure from this lab be used to identify a black ink whose composition is unknown?
Multiple Covalent Bonds
Nitrogen has five valence electrons. If two nitrogen atoms shared a pair of electrons, each one would have only six valence electrons. If they shared two pairs of electrons, each atom would have only seven valence electrons. When the atoms in a nitrogen molecule (N2) share three pairs of electrons, each atom has eight valence electrons. Each pair of shared electrons is represented by a long dash in the structural formula N≡N. When two atoms share three pairs of electrons, the bond is called a triple bond. When two atoms share two pairs of electrons, the bond is called a double bond.

What does the subscript 2 in the formula for a hydrogen molecule indicate?
Unequal Sharing of Electrons
In general, elements on the right of the periodic table have a greater attraction for electrons than elements on the left have (except for noble gases). In general, elements at the top of a group have a greater attraction for electrons than elements at the bottom of a group have. Fluorine is on the far right and is at the top of its group. It has the strongest attraction for electrons and is the most reactive nonmetal.