Figure 9 As a space shuttle lifts off, it leaves a water vapor trail. A reaction of hydrogen and oxygen produces the water.
Using Models How is the bond between hydrogen atoms represented in each model of a hydrogen molecule?
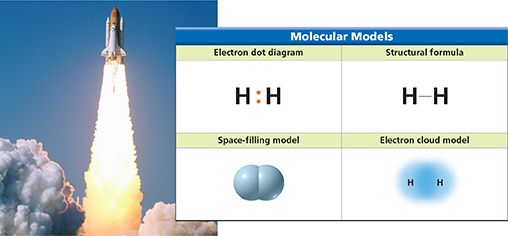
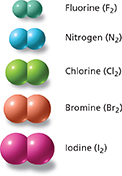
Figure 10 These space-filling models represent diatomic molecules of five elements.
Using Models How many atoms are in a diatomic molecule?
Sharing Electrons
A hydrogen atom has one electron. If it had two electrons, it would have the same electron configuration as a helium atom. Two hydrogen atoms can achieve a stable electron configuration by sharing their electrons and forming a covalent bond. A covalent bond is a chemical bond in which two atoms share a pair of valence electrons. When two atoms share one pair of electrons, the bond is called a single bond.
Figure 9 shows four different ways to represent a covalent bond. In the electron dot model, the bond is shown by a pair of dots in the space between the symbols for the hydrogen atoms. In the structural formula, the pair of dots is replaced by a line. The electron cloud model and the space-filling model show that orbitals of atoms overlap when a covalent bond forms.
Molecules of Elements
Two hydrogen atoms bonded together form a unit called a molecule. A molecule is a neutral group of atoms that are joined together by one or more covalent bonds. The hydrogen molecule is neutral because it contains two protons (one from each atom) and two electrons (one from each atom). What keeps the hydrogen atoms together in the molecule?  The attractions between the shared electrons and the protons in each nucleus hold the atoms together in a covalent bond.
The attractions between the shared electrons and the protons in each nucleus hold the atoms together in a covalent bond.
A chemical formula can be used to describe the molecules of an element as well as a compound. The element hydrogen has the chemical formula H2. The subscript 2 indicates that there are two atoms in a molecule of hydrogen.
Many nonmetal elements exist as diatomic molecules. Diatomic means “two atoms.” Four of the models in Figure 10 are of halogens. A halogen atom has seven valence electrons. If two halogen atoms share a valence electron from each atom, both atoms have eight valence electrons.




