Energy changes in chemical reactions are determined by changes that occur in chemical bonding.  Chemical reactions involve the breaking of chemical bonds in the reactants and the formation of chemical bonds in the products. In the combustion of propane, the bonds in propane and oxygen molecules are broken, while the bonds in carbon dioxide and water molecules are formed.
Chemical reactions involve the breaking of chemical bonds in the reactants and the formation of chemical bonds in the products. In the combustion of propane, the bonds in propane and oxygen molecules are broken, while the bonds in carbon dioxide and water molecules are formed.
Breaking Bonds
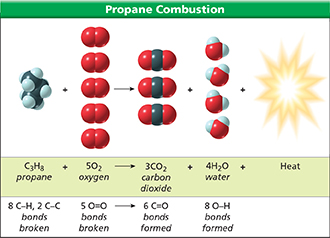
As Figure 17 illustrates, each propane molecule reacts with five oxygen molecules. In order for the reaction to occur, eight CδH single bonds, two CδC single bonds, and five O=O double bonds must be broken. Breaking chemical bonds requires energy. This is why propane grills have an igniter, a device that produces a spark. The spark provides enough energy to break the bonds of reacting molecules and get the reaction started.
Forming Bonds
Figure 17 also shows you that for each molecule of propane burned, three molecules of carbon dioxide and four molecules of water are formed. This means that six C=O double bonds and eight OδH single bonds are formed in the reaction. The formation of chemical bonds releases energy. The heat and light given off by a propane stove result from the formation of new chemical bonds. The bonds form as the carbon, hydrogen, and oxygen atoms in the propane and oxygen molecules are rearranged into molecules of carbon dioxide and water.

Does breaking chemical bonds require energy or release energy?
Figure 17 In order for the combustion of propane to occur, all the chemical bonds in the reactants (propane and oxygen) must be broken. The formation of the chemical bonds in the products completes the reaction.
Inferring How does the chemical energy of the reactants compare to the chemical energy of the products in this reaction?
 d
d



