CHAPTER 1 Assessment
Interactive Textbook with assessment at PHSchool.com

Reviewing Content
Choose the letter that best answers the question or completes the statement.
The application of knowledge to solve practical problems is known as
science.
curiosity.
technology.
experimentation.
Which is not a branch of natural science?
physical science
life science
Earth science
social science
What is the purpose of an experiment?
to communicate data
to test a hypothesis
to prove a scientific law
none of the above
A representation of an object or event is called
a scientific law.
a model.
a hypothesis.
a variable.
Which value is equivalent to ?
five thousand
fifty thousand
five million
fifty million
What is the SI base unit of mass?
gram
kilogram
milligram
pound
An electric generator produces 10 megawatts. This amount is equivalent to
10,000 watts.
1,000,000 watts.
10,000,000 watts.
0.010 watt.
Which of the following is a ratio of equivalent measurements that is used to convert a quantity expressed in one unit to another unit?
slope
conversion factor
derived unit
density
When the ratio of two variables is constant, their relationship can be described as
inversely proportional.
interdependent.
directly proportional.
parallel.
Which of the following would best suit data that describe how a part relates to the whole?
line graph
bar graph
circle graph
scientific notation
Understanding Concepts
Give an example of a case where the branches of natural science appear to overlap.
What is the goal of a scientific method?
How are controlled experiments useful?
Suppose you perform an experiment, and the resulting data do not support your hypothesis. What is the next step you might take?
What are some safety precautions that you should follow when working in the laboratory?
What is scientific notation?
What are the SI base units for length and temperature?
How do derived units differ from base units?
How is the precision of a calculated result related to the precision of the measurements used in the calculation?
How can you convert a temperature expressed in degrees Celsius to kelvins?
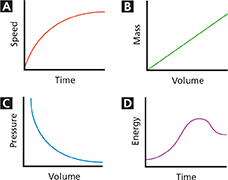
Which of the following graphs describes a direct proportion?
Why is it important for scientists to communicatetheir results?