Rutherford's Atomic Theory
When you try something new, you may have expectations about the outcome. Does the outcome always meet your expectations or are you sometimes surprised? Scientists can also be surprised by the results of their experiments, but unexpected results can lead to important discoveries. This is what happened to Ernest Rutherford (1871–1937).
Rutherford's Hypothesis
In 1899, Ernest Rutherford discovered that uranium emits fast-moving particles that have a positive charge. He named them alpha particles. In 1909, Rutherford asked one of his students, Ernest Marsden, to find out what happens to alpha particles when they pass through a thin sheet of gold.
Recall that in Thomson's model of the atom, the mass and positive charge are evenly spread throughout an atom. Based on this model, Rutherford hypothesized that the mass and charge at any location in the gold would be too small to change the path of an alpha particle. He predicted that most particles would travel in a straight path from their source to a screen that lit up when struck. Those few that did not pass straight through would be deflected only slightly.
The Gold Foil Experiment
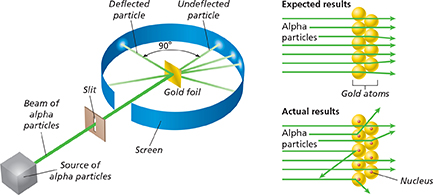
Marsden used the equipment shown in Figure 7. He aimed a narrow beam of alpha particles at the gold. The screen around the gold was made of a material that produced a flash of light when struck by a fast-moving alpha particle. By observing the flash, Marsden could figure out the path of an alpha particle after it passed through the gold.
Some of the locations of the flashes on the screen did not support Rutherford's prediction. More particles were deflected than he expected. About one out of every 20,000 was deflected by more than 90 degrees. Some of the alpha particles behaved as though they had struck an object and bounced straight back.
Figure 7 The path of an alpha particle can be detected by the location of a flash on a screen. Rutherford expected the paths of the positively charged alpha particles that were aimed at the thin gold foil to be affected only slightly by the gold atoms. But more particles were deflected than expected and some particles bounced straight back.
 dd
dd




