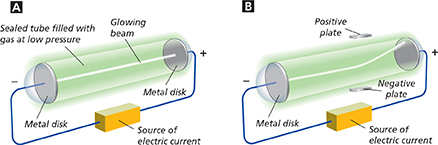
Figure 5 Thomson used a sealed tube of gas in his experiments. A When the current was on, the disks became charged and a glowing beam appeared in the tube. B The beam bent toward a positively charged plate placed outside the tube.
Inferring What was the charge on the particles in the beam?
 dd
ddEvidence for Subatomic Particles
Thomson concluded that the particles in the beam had a negative charge because they were attracted to the positive plate. He hypothesized that the particles came from inside atoms. He had two pieces of evidence to support his hypothesis. No matter what metal Thomson used for the disk, the particles produced were identical. The particles had about the mass of a hydrogen atom, the lightest atom.
Thomson's discovery changed how scientists thought about atoms. Before his experiments, the accepted model of the atom was a solid ball of matter that could not be divided into smaller parts.  Thomson's experiments provided the first evidence that atoms are made of even smaller particles. Thomson revised Dalton's model to account for these subatomic particles.
Thomson's experiments provided the first evidence that atoms are made of even smaller particles. Thomson revised Dalton's model to account for these subatomic particles.
Thomson's Model
An atom is neutral, meaning it has neither a negative nor a positive charge. How can an atom contain negative particles and still be neutral? There must be some positive charge in the atom. In Thomson's model of the atom, the negative charges were evenly scattered throughout an atom filled with a positively charged mass of matter. The model is called the “plum pudding” model, after a traditional English dessert.
You might prefer to think of Thomson's model as the “chocolate chip ice cream” model. Think of the chocolate chips in Figure 6 as the negative particles and the vanilla ice cream as the positively charged mass of matter. When the chocolate chips are spread evenly throughout the ice cream, their “negative charges” balance out the “positive charge” of the vanilla ice cream.

How do objects with the same charge behave when they come close to one another?
Figure 6 A scoop of chocolate chip ice cream can represent Thomson's model of the atom. The chips represent negatively charged particles, which are spread evenly through a mass of positively charged matter—the vanilla ice cream.





