Quick Lab

Comparing Excited States
Materials
fluorescent (“neon”) markers, glow-in-the-dark toy, ultraviolet (UV) lamp
Procedure
Use the fluorescent markers to draw a picture on a piece of paper.
With the room darkened, observe your drawing under a UV lamp. CAUTION Do not look directly at the light. Remove the drawing from under the UV light and observe it again. Record your observations.
Observe the glow-in-the-dark toy under the UV light. Remove the toy from the light and observe it again. Record your observations.
Analyze and Conclude
Observing How did the glow of the toy differ from the glow of your drawing?
Formulating Hypotheses Use the concepts of ground and excited states to explain how UV light caused your drawing and the toy to glow.
Drawing Conclusions In which object, your drawing or the toy, do the atoms have excited states that are more stable, or less likely to change? Explain your answer.
Atomic Orbitals
The electron cloud represents all the orbitals in an atom. An orbital is a region of space around the nucleus where an electron is likely to be found. To understand the concept of an orbital, imagine a map of your school. Suppose you mark your exact location with a dot once every 10 minutes over a period of one week. The places you visit the most— such as your classrooms, the cafeteria, and the area near your locker—would have the highest concentration of dots. The places you visit the least would have the lowest concentration of dots.
The dots on your map are a model of your “orbital.” They describe your most likely locations. There are some locations in your orbital that you may not visit every week—such as the principal's office or the auditorium. These locations may not be represented by a dot on your map. Despite such omissions, the dots on your map are a good model of how you usually behave in your orbital.  An electron cloud is a good approximation of how electrons behave in their orbitals.
An electron cloud is a good approximation of how electrons behave in their orbitals.
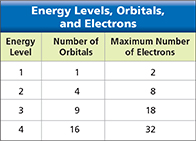
The level in which an electron has the least energy—the lowest energy level—has only one orbital. Higher energy levels have more than one orbital. Figure 15 shows the number of orbitals in the first four energy levels of an atom. Notice that the maximum number of electrons in an energy level is twice the number of orbitals. Each orbital can contain two electrons at most.
Figure 15 The table lists the number of orbitals in the first four energy levels of an atom. It also lists the maximum number of electrons in each energy level. Inferring How many electrons can be in each orbital?
 d
d



