Beta Decay
When thorium-234 decays, it releases negatively charged radiation called beta particles. A beta particle is an electron emitted by an unstable nucleus. In nuclear equations, a beta particle is written as or β. Because of its single negative charge, a beta particle is assigned an atomic number of ‒1. In Chapter 4, you learned that an electron has very little mass when compared with a proton. For this reason, a beta particle is assigned a mass number of 0.
How can an atomic nucleus, which has a positive charge, emit a negatively charged particle? During beta decay, a neutron decomposes into a proton and an electron. The proton stays trapped in the nucleus, while the electron is released. The following equation describes the beta decay of thorium-234.
In beta decay, the product isotope has one proton more and one neutron fewer than the reactant isotope. The mass numbers of the isotopes are equal because the emitted beta particle has essentially no mass.
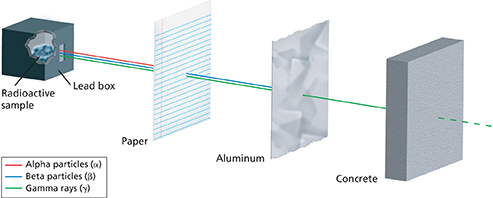
Due to their smaller mass and faster speed, beta particles are more penetrating than alpha particles. As Figure 4 illustrates, beta particles pass through paper, but can be stopped by a thin sheet of metal.

What is a beta particle?
Gamma Decay
Not all nuclear radiation consists of charged particles. A gamma ray is a penetrating ray of energy emitted by an unstable nucleus. The symbol for a gamma ray is γ. Gamma radiation has no mass and no charge. Like X-rays and visible light, gamma rays are energy waves that travel through space at the speed of light.
Figure 4 Alpha particles (shown in red) are the least penetrating type of nuclear radiation. Gamma rays (shown in green) are the most penetrating. A concrete slab can block most but not all of the gamma rays released by a radioactive source.
Interpreting Diagrams Which type of radiation can penetrate paper but is blocked by aluminum foil?
 d
d




