Figure 3 Microorganisms in the stomachs of a cow produce more than 500 liters of methane (CH4) per day.

Saturated Hydrocarbons
Grass contains a compound called cellulose. Most organisms, including the grazing cows in Figure 3, cannot digest cellulose. However, microorganisms in cows' stomachs break down cellulose into smaller molecules that cows can digest. One of the products of this process is methane, CH4, which is a hydrocarbon. A hydrocarbon is an organic compound that contains only the elements hydrogen and carbon. Methane is a saturated hydrocarbon. In a saturated hydrocarbon, all of the bonds are single bonds. A saturated hydrocarbon contains the maximum possible number of hydrogen atoms for each carbon atom. Another name for a saturated hydrocarbon is an alkane. Names of alkane compounds end in −ane, as in methane and propane.
 Factors that determine the properties of a hydrocarbon are the number of carbon atoms and how the atoms are arranged. A hydrocarbon molecule can contain one carbon atom, as in methane, or more than 30 carbon atoms, as in asphalt. The carbon atoms can be arranged in a straight chain, a branched chain, or a ring.
Factors that determine the properties of a hydrocarbon are the number of carbon atoms and how the atoms are arranged. A hydrocarbon molecule can contain one carbon atom, as in methane, or more than 30 carbon atoms, as in asphalt. The carbon atoms can be arranged in a straight chain, a branched chain, or a ring.
Straight Chains
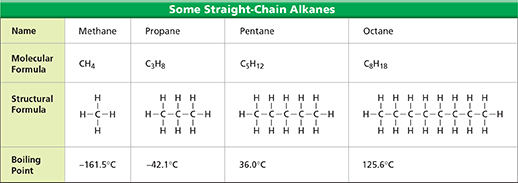
Figure 4 lists the names, molecular formulas, structural formulas, and boiling points for four straight-chain alkanes. Recall that a molecular formula shows the type and number of atoms in a molecule of the compound. A structural formula shows how those atoms are arranged. The number of carbon atoms in a straight-chain alkane affects the state of the alkane at room temperature. Methane and propane are gases. Pentane and octane are liquids. The more carbon atoms, the higher the boiling point is.

What is another name for a saturated hydrocarbon?
Figure 4 Molecules of ethane, propane, pentane, and octane have two, three, five, and eight carbon atoms, respectively. Making Generalizations How does increasing the number of carbon atoms affect the boiling point of a straight-chain alkane?
 dd
dd



