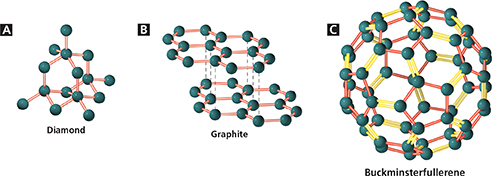
Figure 2 There are several forms of carbon. A In diamond, carbon atoms are arranged in a rigid, three-dimensional network. B In graphite, the atoms are arranged in layers. C In fullerenes, carbon atoms are arranged in hollow spheres. Predicting Are the properties of fullerenes more like those of diamond or of graphite? Give a reason for your choice.
Forms of Carbon
The element carbon exists in several forms with different properties.  Diamond, graphite, and fullerenes are forms of carbon. In each form, there is a different arrangement of bonded carbon atoms.
Diamond, graphite, and fullerenes are forms of carbon. In each form, there is a different arrangement of bonded carbon atoms.
Diamond
Cutting, grinding, and drilling tools are often coated with diamond because no substance is harder than diamond. Diamond is an example of a network solid. In a network solid, all the atoms are linked by covalent bonds. A network solid is sometimes described as a single molecule. Figure 2A shows how the carbon atoms are linked in diamond. Covalent bonds connect each carbon atom in diamond to four other carbon atoms. The three-dimensional structure that results is rigid, compact, and strong. Diamond is harder than other substances because cutting a diamond requires breaking many covalent bonds.
Graphite
A second form of carbon, graphite, has very different properties from diamond. It is extremely soft and slippery. Figure 2B shows how the carbon atoms in graphite are arranged in widely spaced layers. Within each layer, each carbon atom forms strong covalent bonds with three other carbon atoms. Between graphite layers the bonds are weak, which allows the layers to slide easily past one another. Because graphite is soft and slippery, it is a good lubricant for moving metal parts in machinery. Pencil “lead” is a mixture of graphite and clay. The layered structure of graphite explains why you can make pencil marks on paper and why the marks can be erased easily.
Fullerenes
In 1985, researchers discovered a third form of carbon in the soot produced when some carbon compounds burn. Fullerenes are large hollow spheres, or cages, of carbon. These cages have since been found in meteorites. Figure 2C shows a model of a cage made from 60 carbon atoms. On the surface of the cage, the atoms form alternating hexagons and pentagons, like a soccer ball cover. The C60 molecule is called buckminsterfullerene after Buckminster Fuller, an architect who designed domes with similar geometric patterns.





