Mendeleev's Periodic Table
In the 1860s, Mendeleev was working on a textbook to use with his chemistry students. Because he needed to describe 63 elements, Mendeleev was looking for the best way to organize the information. He found a way to approach the problemwhile playing his favorite card game, a version ofsolitaire. In this game, the player sorts a deck of cards by suit and value. To finish the game, the player must end up with four columns, as shown in Figure 2. Each column contains cards of a single suit arranged in order by value.
Figure 2 A deck of cards can be divided into four suits—diamonds, spades, hearts, and clubs. In one version of solitaire, a player must produce an arrangement in which each suit is ordered from ace to king. This arrangement is a model for Mendeleev's periodic table.

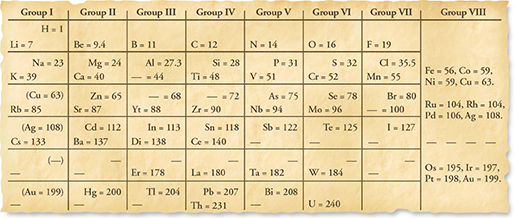
Mendeleev's Proposal Mendeleev's strategy for organizing the elements was modeled on the card game. Mendeleev made a “deck of cards” of the elements. On each card, he listed an element's name, mass, and properties. He paid special attention to how each element behaved in reactions with oxygen and hydrogen. When Mendeleev lined up the cards in order of increasing mass, a pattern emerged. The key was to break the elements into rows, as shown in Figure 3.
 Mendeleev arranged the elements into rows in order of increasing mass so that elements with similar properties were in the same column. The final arrangement was similar to a winning arrangement in solitaire, except that the columns were organized by properties instead of suits. Within a column, the masses increased from top to bottom. Mendeleev's chart was a periodic table. A periodic table is an arrangement of elements in columns, based on a set of properties that repeat from row to row.
Mendeleev arranged the elements into rows in order of increasing mass so that elements with similar properties were in the same column. The final arrangement was similar to a winning arrangement in solitaire, except that the columns were organized by properties instead of suits. Within a column, the masses increased from top to bottom. Mendeleev's chart was a periodic table. A periodic table is an arrangement of elements in columns, based on a set of properties that repeat from row to row.
Figure 3 This is a copy of a table that Mendeleev published in1872. He placed the elements in groups based on the compounds they formed with oxygen or hydrogen. Using Tables How many elements did Mendeleev place in Group II?