Appendices
Appendix A SI Units
SI (Système International d'Unités) is a revised version of the metric system, which was originally developed in France in 1791. SI units of measurement are used by scientists throughout the world. The system is based on multiples of ten. Each unit is ten times larger or ten times smaller than the next unit. The most commonly used SI units are given below.
You can use conversion factors to convert between SI and non-SI units. Try the following conversions. How tall are you in meters? What is your weight in newtons? What is your normal body temperature in degrees Celsius?
Metric ruler

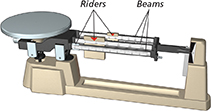
Triple-Beam Balance
Length |
The distance from one point to another |
meter (m) |
A meter is slightly longer than a yard. 1 meter = 1000 millimeters (mm) 1 meter = 100 centimeters (cm) 1000 meters = 1 kilometer (km) |
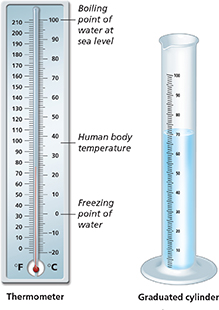
Volume |
The amount of space an object takes up |
liter (L) |
A liter is slightly more than a quart. 1 liter = 1000 milliliters (mL) |
Mass |
The amount of matter in an object |
gram (g) |
A gram has a mass equal to about one paper clip. 1000 grams = 1 kilogram (kg) |
Temperature |
The measure of hotness or coldness |
degrees |
0°C = freezing point of water at sea level |
Celsius (°C) |
100°C = boiling point of water at sea level |
Metric–Customary Equivalents
2.54 centimeters (cm) = 1 inch (in.)
1 meter (m) = 39.37 inches (in.)
1 kilometer (km) = 0.62 miles (mi)
1 liter (L) = 1.06 quarts (qt)
250 milliliters (mL) = 1 cup (c)
9.8 newtons (N) = 2.2 pounds (lb)




