CHAPTER 2 Study Guide
2.1 Classifying Matter
 Key Concepts
Key Concepts
Every sample of a given substance has the same properties because a substance has a fixed, uniform composition.
An element has a fixed composition because it contains only one type of atom.
A compound always contains two or more elements joined in a fixed proportion.
The properties of a mixture can vary because the composition of a mixture is not fixed.
Based on the size of its largest particles, a mixture can be classified as a solution, a suspension, or a colloid.
Vocabulary
pure substance, p. 39
element, p. 39
atom, p. 39
compound, p. 40
heterogeneous mixture, p. 41
homogeneous mixture, p. 42
solution, p. 42
suspension, p. 43
colloid, p. 44
2.2 Physical Properties
 Key Concepts
Key Concepts
Viscosity, conductivity, malleability, hardness, melting point, boiling point, and density are examples of physical properties.
Physical properties are used to identify a material, to choose a material for a specific purpose, or to separate the substances in a mixture.
Filtration and distillation are two common separation methods.
Vocabulary
physical property, p. 45
viscosity, p. 45
conductivity, p. 46
malleability, p. 46
melting point, p. 47
boiling point, p. 47
filtration, p. 50
distillation, p. 50
physical change, p. 51
2.3 Chemical Properties
 Key Concepts
Key Concepts
Chemical properties can be observed only when the substances in a sample of matter are changing into different substances.
Three common types of evidence for a chemical change are a change in color, the production of a gas, and the formation of a precipitate.
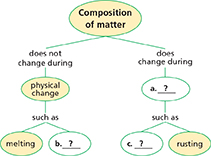
When matter undergoes a chemical change, the composition of the matter changes. When matter undergoes a physical change, the composition of the matter remains the same.
Vocabulary
chemical property, p. 54
flammability, p. 54
reactivity, p. 55
chemical change, p. 56
precipitate, p. 57
Thinking Visually
Concept Map Use information from the chapter to complete the concept map below.