Hardness
One way to compare the hardness of two materials is to see which of the materials can scratch the other. The blade of a typical kitchen knife, for example, can scratch a copper sheet because stainless steel is harder than copper. The stainless steel in a knife blade is a hard solid that can be shaped into a sharp cutting edge. The material used to sharpen the blade must be harder than stainless steel. Diamond is the hardest known material. Some of the grinding wheels used to sharpen steel contain small grains of diamond. The man in Figure 11 is carving a canoe from a soft wood—Western red cedar.
Figure 11 This Tlingit carver is using an adze to carve a canoe from Western red cedar. Red cedar is a relatively soft wood.

Melting and Boiling Points
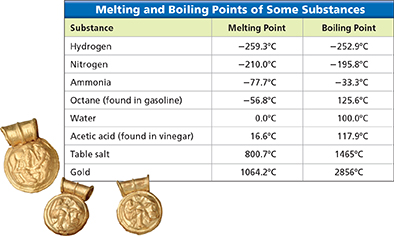
If yo leave a tray of ice cubes on your kitchen counter, the ice cubes will melt. The temperature at which a substance changes from solid to liquid is its melting point. For water, this change normally occurs at 0°C. If yo heat water to cook pasta at sea level, the water will start to boil at 100°C. The temperature at which a substance boils is its boiling point. Figure 12 shows the melting point and the boiling point for some substances.
Density
Density can be used to test the purity of a substance. Recall that density is the ratio of the mass of a substance to its volume. At room temperature, silver has a density of 10.5 g/cm3. If a coin has a density of 9.9 g/cm3 at room temperature, either the coin is not made from silver or the coin contains substances in addition to silver.
Density can be used to test the purity of methanol. Methanol is a fuel burned in some racing motorcycles. The American Motorcycle Association (AMA) requires racers to use fuel that is at least 99.65 percent pure. Race officials may collect a sample of fuel and measure its temperature and density. Then they compare the measured density to the expected density of methanol at that temperature. These spot checks keep racers from adding substances to the fuel that will give them an unfair advantage in a race.
Figure 12 The table lists the melting points and boiling points for several substances.
Analyzing Data Which of these substances are liquids at room temperature (20ºC, or 68ºF)?
 dd
dd



