Temperature
How do you know something is hot? You might use a thermometer to measure its temperature. Temperature is a measure of how hot or cold an object is compared to a reference point. Recall that on the Celsius scale, the reference points are the freezing and boiling points of water. On the Kelvin scale, another reference point is absolute zero, which is defined as a temperature of 0 kelvins.
 Temperature is related to the average kinetic energy of the particles in an object due to their random motions through space. As an object heats up, its particles move faster, on average. As a result, the average kinetic energy of the particles, and the temperature, must increase.
Temperature is related to the average kinetic energy of the particles in an object due to their random motions through space. As an object heats up, its particles move faster, on average. As a result, the average kinetic energy of the particles, and the temperature, must increase.
Why does heat flow from a high to a low temperature? One way that heat flows is by the transfer of energy in collisions. On average, high-energy particles lose energy, and low-energy particles gain energy in collisions. Overall, collisions transfer thermal energy from hot to cold objects.
Thermal Energy
Recall that thermal energy is the total potential and kinetic energy related to the motion of all the particles in an object.
 Thermal energy depends on the mass, temperature, and phase (solid, liquid, or gas) of an object.
Thermal energy depends on the mass, temperature, and phase (solid, liquid, or gas) of an object.
Thermal energy, unlike temperature, depends on mass. Suppose you compare a cup of tea and a teapot full of tea. Both are at the same temperature, so the average kinetic energy of the particles is the same in both containers. However, there is more thermal energy in the teapot because it contains more particles.
Now consider how thermal energy varies with temperature. You can do this by comparing a cup of hot tea with a cup of cold tea. In both cases, the tea has the same mass, and the same number of particles. But the average kinetic energy of particles is higher in the hot tea, so it also has greater thermal energy than the cold tea.
Figure 2 shows the particles in a cup of hot tea and in a pitcher of lemonade. The tea is at a higher temperature because its particles move a little faster, on average. But they are only moving slightly faster, and the pitcher of lemonade has many more particles than the tea. As it turns out, the pitcher of lemonade has more thermal energy than the cup of hot tea.
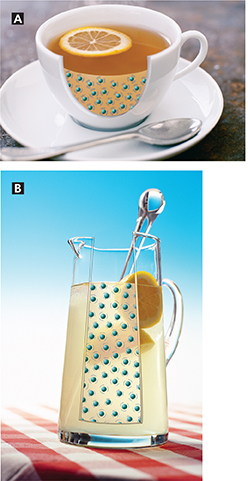
Figure 2 Thermal energy depends on mass and temperature. A The tea is at a higher temperature than the lemonade because its particles have a higher average kinetic energy.
B The lemonade is at a lower temperature, but it has more thermal energy because it has many more particles. Inferring In which liquid are water particles moving faster, on average?





