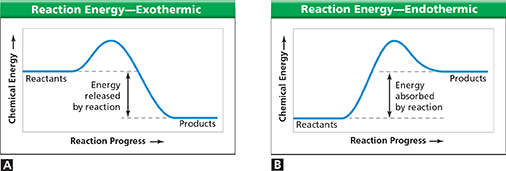
Figure 18 In chemical reactions, energy is either released or absorbed. A In an exothermic reaction, energy is released to the surroundings. B In an endothermic reaction, energy is absorbed from the surroundings.
Using Graphs How do the energy diagrams show that energy is conserved in chemical reactions?
 dd
ddExothermic and Endothermic Reactions
Recall that physical changes can release or absorb energy. During an exothermic change, such as freezing, energy is released to the surroundings. During an endothermic change, such as melting, energy is absorbed from the surroundings. Energy also flows into and out of chemical changes.  During a chemical reaction, energy is either released or absorbed.
During a chemical reaction, energy is either released or absorbed.
Exothermic Reactions
A chemical reaction that releases energy to its surroundings is called an exothermic reaction. In exothermic reactions, the energy released as the products form is greater than the energy required to break the bonds in the reactants.
Combustion is an example of an extremely exothermic reaction. When 1 mole of propane reacts with 5 moles of oxygen, 2220 kJ (kilojoules) of heat is released. You can use this value to replace “heat” in the combustion equation written earlier.
Figure 18A shows how chemical energy changes during an exothermic reaction. Notice that the chemical energy of the reactants is greater than the chemical energy of the products. The difference between these amounts of energy equals the amount of heat given off by the reaction.
In any reaction, the chemical energy reaches a peak before the reactants change into products. This peak represents the amount of energy required to break the chemical bonds of the reactants. Unless reacting particles collide with enough energy to break these bonds, the reaction will not occur. For example, at room temperature, the collisions between propane and oxygen molecules are not energetic enough to result in combustion. However, if you increase the temperature by adding a spark, some of the molecules around the spark move faster and are able to collide with enough energy to react.




