Double Replacement
A double-replacement reaction is one in which two different compounds exchange positive ions and form two new compounds. The general form of a double replacement reaction is
Notice that two replacements take place in this reaction. Not only is A replacing C, but C is also replacing A.
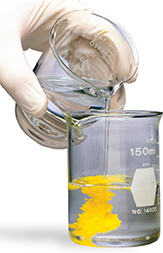
Solutions of lead(II) nitrate, Pb(NO3)2, and potassium iodide, KI, are both colorless. However, when these solutions are mixed, as shown in Figure 13, a yellow precipitate forms as a result of a double-replacement reaction. The equation for this reaction is
The lead ions in Pb(NO3)2, trade places with the potassium ions in KI. The products are lead(II) iodide, PbI2, which precipitates out of solution, and potassium nitrate, KNO3, which remains in solution.
When geologists test the calcium carbonate content in a rock, they make use of the following double-replacement reaction.
One of the products of this reaction is calcium chloride, CaCl2. The other product is carbonic acid, H2CO3, which decomposes into carbon dioxide gas and water.
Figure 13 When potassium iodide solution is poured into a solution of lead(II) nitrate, a double-replacement reaction takes place. Lead(II) iodide forms as a yellow precipitate.
Comparing and Contrasting How does a double-replacement reaction differ from a single-replacement reaction?
Quick Lab
Identifying a Type of Reaction
Materials
piece of zinc, copper(II) sulfate (CuSO4) solution, 250-mL beaker, tongs, paper towel
Procedure 
Place the zinc in the beaker and add enough CuSO4 solution to cover the zinc as shown. CAUTION Be careful when using chemicals. Copper sulfate is toxic.
After 5 minutes, carefully remove the zinc from the solution using the tongs and place the zinc on the paper towel to dry. Observe any changes that have occurred to the zinc and the solution of CuSO4. CAUTION Follow your teacher's instructions for disposal of used chemicals. Wash your hands with soap or detergent before leaving the laboratory.

Analyze and Conclude
Observing What clues indicate that a chemical reaction has taken place?
Applying Concepts What were the reactants in this reaction? What were the products? Write a balanced chemical equation for the reaction.
Classifying Is this a single-replacement or double-replacement reaction? Explain.




