Catalysts Sometimes you can change a reaction rate by using catalysts. A catalyst is a substance that affects the reaction rate without being used up in the reaction. Chemists often use catalysts to speed up a reaction or enable a reaction to occur at a lower temperature. In the making of sulfuric acid, one of the steps involved is the reaction of sulfur dioxide with oxygen to form sulfur trioxide. This reaction happens very slowly without a catalyst such as vanadium(V) oxide.
Since the catalyst is neither a reactant nor a product, it is written over the arrow. Because the catalyst is not consumed, it can be used to speed up the same reaction over and over again.
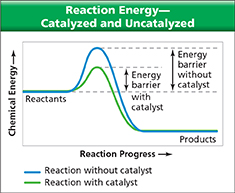
Recall that in order for a reaction to take place, the reacting particles must collide with enough energy to break the chemical bonds of those particles. As shown in Figure 23, a catalyst lowers this energy barrier. One way that a catalyst can do this is by providing a surface on which the reacting particles can come together. Imagine that you go to a party and make several new friends. By bringing people together, the party has made it easier for you to form those friendships. Similarly, a catalyst can “invite” reacting particles together so that they are more likely to react.
Figure 23 The graph above shows how a catalyst lowers the amount of energy required for effective collisions between reacting particles.
Using Graphs In an exothermic reaction, how does a catalyst affect the amount of energy released?
 d
dSection 7.4 Assessment
Reviewing Concepts
 What does a reaction rate tell you?
What does a reaction rate tell you? What five factors affect reaction rates?
What five factors affect reaction rates?Explain why reactions take place faster at higher temperatures.
When you add baking soda to vinegar, the mixture fizzes as carbon dioxide gas is produced. Suppose you added water to the vinegar before you mixed it with the baking soda. What do you think would happen to the rate of carbon dioxide production?
How does a catalyst make a reaction go faster?
Platinum is a catalyst for the decomposition of hydrogen peroxide into water and oxygen.
What would you expect to see if platinum were added to hydrogen peroxide solution?
Critical Thinking
Applying Concepts Explain why, if you want to store uncooked hamburger meat for a month, you put it in a freezer rather than a refrigerator.
Evaluating The reaction between magnesium and hydrochloric acid produces hydrogen. If you increase the concentration of HCl, the reaction takes place faster. Could HCl be considered a catalyst for this reaction? Explain your answer.
Writing in Science
Compare and Contrast Paragraph Write a paragraph explaining how temperature, concentration, surface area, and catalysts affect reaction rates.




