Data Analysis
What Determines the Size of an Atom or Ion?
Scientists use atomic radii to compare the sizes of atoms of different elements. Remember from mathematics that the radius of a sphere is the distance from the center of the sphere to its outer edge. The radius is half the diameter of the sphere. Because atomic radii are extremely small, these distances are expressed in units called picometers (pm). As a comparison, there are one billion (109) picometers in a millimeter.
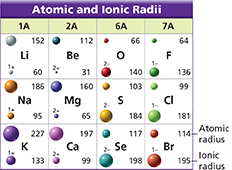
The table shows the atomic radius and ionic radius for six metals and six nonmetals. You will use the data to relate the size of an element's atoms to the element's location on the periodic table. You also will use the data to compare the sizes of atoms and their ions.
Using Tables Within a period, what happens to the atomic radius as the atomic number of the elements increases?
Using Tables Within Groups 1A, 2A, 6A, and 7A, what happens to the atomic radius of elements as the atomic number increases?
Inferring How does adding an occupied energy level affect the atomic radius? (Hint: Lithium is a Period 2 element and sodium is a Period 3 element.)
Comparing and Contrasting Compare the atomic and ionic radii for potassium (K), and for bromine (Br).
Making Generalizations What happens to the radius of an atom when the atom loses electrons? When the atom gains electrons?
Relating Cause and Effect Explain the difference in size between a metal atom and its cation.
The ion that forms when a chlorine atom gains an electron has 17 protons and 18 electrons. This ion has a charge of 1– because it has one extra electron. The symbol for the ion is written Cl1−, or Cl− for short. An ion with a negative charge is an anion (AN eye un). Anions like the Cl− ion are named by using part of the element name plus the suffix –ide. Thus, Cl− is called a chloride ion.
A sodium ion has 11 protons and 10 electrons. Because it has one extra proton, the sodium ion has a charge of 1+. The symbol for the ion is written Na1+, or Na+ for short. An ion with a positive charge is a cation (KAT eye un). Naming a cation is easy. You just use the element name, as in the sodium ion.
Formation of Ionic Bonds
Remember that a particle with a negative charge will attract a particle with a positive charge. When an anion and a cation are close together, a chemical bond forms between them. A chemical bond is the force that holds atoms or ions together as a unit. An ionic bond is the force that holds cations and anions together. An ionic bond forms when electrons are transferred from one atom to another.