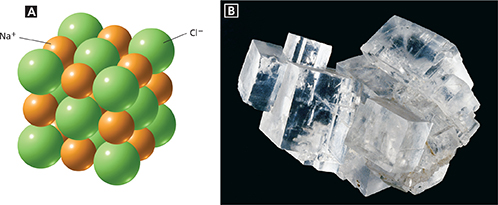
Figure 5 The structure and shape of a crystal are related. A In a sodium chloride crystal, each ion is surrounded by six oppositely charged ions. B Sodium chloride crystals are shaped like cubes.
Crystal Lattices
A chemical formula for an ionic compound tells you the ratio of the ions in the compound. But it does not tell you how the ions are arranged in the compound. If you looked at a sample of sodium chloride with a hand lens or microscope, you would be able to see that the pieces of salt are shaped like cubes. This shape is a clue to how the sodium and chloride ions are arranged in the compound.
Figure 5A shows that the ions in sodium chloride are arranged in an orderly, three-dimensional structure. Each chloride ion is surrounded by six sodium ions and each sodium ion is surrounded by six chloride ions. Each ion is attracted to all the neighboring ions with an opposite charge. This set of attractions keeps the ions in fixed positions in a rigid framework, or lattice. The repeating pattern of ions in the lattice is like the repeating pattern of designs on the wallpaper in Figure 6.
Solids whose particles are arranged in a lattice structure are called crystals. Compare the cubic shape of the sodium chloride crystals in Figure 5B to the arrangement of ions in Figure 5A. The shape of an ionic crystal depends on the arrangement of ions in its lattice. In turn, the arrangement of the ions depends on the ratio of ions and their relative sizes. Crystals are classified into groups based on the shape of their crystals. Crystals of ruby have a six-sided, hexagonal shape. The How It Works box on page 163 describes one way to make rubies.

What shape are sodium chloride crystals?
Figure 6 This wallpaper displays a repeating pattern of flower and fruit designs. Using Analogies How is this arrangement of designs similar to the arrangement of ions in a crystal?





