Dispersion of Molecular Compounds
When you place a piece of hard candy on your tongue, the sweet taste spreads, or disperses, throughout your mouth. The water in your saliva dissolves the sugar and flavoring in the candy. Sugar dissolves in water by dispersion, or breaking into small pieces that spread throughout the water.
Both sugar and water are polar molecules, so they attract one another. Because the water molecules are constantly moving, they collide frequently with the surface of the sugar crystals, as shown in Figure 4. Attractions form between the water molecules and the exposed sugar molecules. When enough water molecules have surrounded a sugar molecule, the attractions between them are great enough to overcome the attractions holding the sugar molecule to the surface of the crystal. The sugar molecule breaks free, and is pulled into solution by the water molecules.
As more sugar molecules break free of the crystal, another layer of sugar molecules is exposed to the water, and the process repeats. The solute particles become evenly spread throughout the solvent.
Figure 4 Saliva dissolves the sugar in hard candy by dispersion. As water molecules collide with sugar crystals, attractions develop between the water molecules and sugar molecules at the surface of the solid.
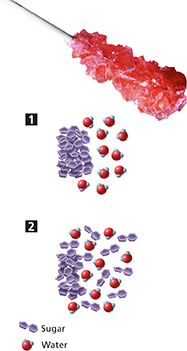

How does sugar dissolve in water?
Ionization of Molecular Compounds
Hydrogen chloride, HCl, is a molecular compound in which a hydrogen atom and a chlorine atom share a pair of electrons. Recall that a hydrogen atom has only one proton and one electron. When HCl gas dissolves in water, the hydrogen proton from each HCl molecule is transferred to a water molecule. For each HCl molecule that reacts, a hydronium ion, H3O+, and a chloride ion, Cl−, are produced.
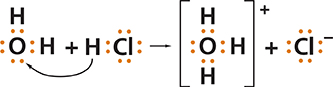
Notice that when hydrogen chloride and water form a solution, two molecular compounds react to form two ions. The process in which neutral molecules gain or lose electrons is known as ionization. Unlike dissociation and dispersion, which are physical changes, dissolving by ionization is a chemical change. The solution that results contains new substances. When a solute dissolves by dissociation, the ions pulled into solution are the same ions present in the solute. When a solute dissolves by ionization, the ions in solution are formed by the reaction of solute and solvent particles.




