Neutralization and Salts
When people eat fish, they sometimes squeeze lemon juice over the fish. Fish contains bases that can leave a bitter taste. Lemon juice contains acids, such as citric acid. By squeezing lemon juice over the fish, the citric acid reacts with the bases in the fish, and the fish tastes less bitter.
The reaction between an acid and a base is called neutralization. During neutralization, the negative ions in an acid combine with the positive ions in a base to produce an ionic compound called a salt. At the same time, the hydronium ions from the acid combine with the hydroxide ions from the base to produce water.  The neutralization reaction between an acid and a base produces a salt and water.
The neutralization reaction between an acid and a base produces a salt and water.
For example, when hydrochloric acid reacts with sodium hydroxide, the following neutralization reaction occurs.
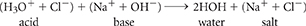
The products of the reaction are a salt made up of sodium and chloride ions, and water. If you let the water in the resulting solution evaporate, the sodium and chloride ions would begin to crystallize out of solution, forming table salt.
Table salt is the most common example of a salt compound. Other common salts are listed in Figure 19. For instance, baking soda, NaHCO3, is produced during the neutralization reaction between sodium hydroxide and carbonic acid, H2CO3. The other product is water. The ocean contains many dissolved salts, including chlorides and sulfates of potassium, calcium, magnesium, and sodium. Many of these salts go into solution as seawater washes against rocks.
| Common Salts | ||
|---|---|---|
Name | Formula | Uses |
Sodium chloride | NaCl | Food flavoring, preservative |
Sodium carbonate | Na2CO3 | Used to make glass |
Potassium chloride | KCl | Used as a salt substitute to reduce dietary intake of sodium |
Potassium iodide | KI | Added to table salt to prevent iodine deficiency |
Magnesium chloride | MgCl2 | De-icer for roads |
Calcium carbonate | CaCO3 | Chalk, marble floors, and tables |
Ammonium nitrate | NH4NO3 | Fertilizer, cold packs |
Figure 19 The common salts listed in the table can all be made by reacting an acid with a base. One of these salts, sodium carbonate, was used to make the glass for the vases shown below. Inferring Name an acid and a base that could react to form potassium chloride, KCl.





