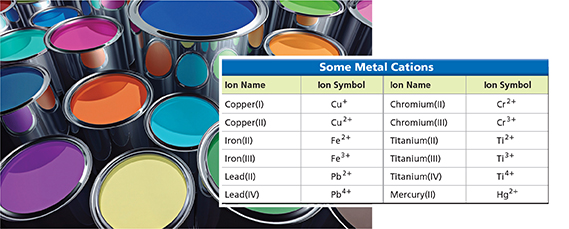
Figure 17 Many paint pigments contain compounds of transition metals. These metals often form more than one type of ion. The ion names must contain a Roman numeral. Using Tables How is the Roman numeral in the name related to the charge on the ion?
 dd
ddMetals With Multiple Ions
The alkali metals, alkaline earth metals, and aluminum form ions with positive charges equal to the group number. For example, the symbol for a potassium ion is K+, the symbol for a calcium ion is Ca2+, and the symbol for an aluminum ion is Al3+.
Many transition metals form more than one type of ion. Notice the two copper ions listed in Figure 17, a copper(I) ion with a 1+ charge and a copper(II) ion with a 2+ charge. When a metal forms more than one ion, the name of the ion contains a Roman numeral to indicate the charge on the ion. These ion names can distinguish red copper(I) oxide from black copper(II) oxide. The formula for “copper one oxide” is Cu2O because it takes two Cu1+ ions to balance the charge on an O2− ion. The formula for “copper two oxide” is CuO because it takes only one Cu2+ ion to balance the charge on an O2− ion.
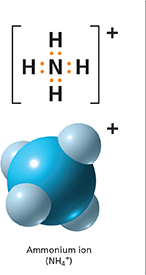
Figure 18 The atoms in an ammonium ion are joined by covalent bonds. The ion loses a valence electron as it forms. This loss leaves only 10 electrons to balance the charge on 11 protons.
Polyatomic Ions
The electron dot diagram in Figure 18 describes a group of atoms that includes one nitrogen and four hydrogen atoms. It is called an ammonium ion. The atoms are joined by covalent bonds. Why does the group have a positive charge? The nitrogen atom has seven protons, and each hydrogen atom has one proton—eleven in total. But the group has only ten electrons to balance the charge on the protons—eight valence electrons and nitrogen's two inner electrons.
A covalently bonded group of atoms that has a positive or negative charge and acts as a unit is a polyatomic ion. The prefix poly-means “many.” Most simple polyatomic ions are anions. Figure 19 lists the names and formulas for some polyatomic ions. Sometimes there are parentheses in a formula that includes polyatomic ions. For example, the formula for iron(III) hydroxide is Fe(OH)3. The subscript 3 indicates that there are three hydroxide ions for each iron(III) ion.

When are Roman numerals used in compound names?




