Quick Lab
Modeling Molecules
Materials
blue plastic-foam ball, black plastic-foam ball, 7 white gumdrops, toothpicks

Procedure
To make a model of an ammonia molecule (NH3), insert a toothpick in each of 3 gumdrops. The gumdrops represent hydrogen atoms and the toothpicks represent bonds.
An ammonia molecule is like a pyramid with the nitrogen at the top and the hydrogen atoms at the corners of the base. Insert the toothpicks in the blue foam ball (nitrogen) so that each gumdrop is the same distance from the ball.
The hydrogen atoms in a methane molecule (CH4) are equally spaced around the carbon. Use the black ball to make a model of methane.
Analyze and Conclude
Comparing and Contrasting Compare the shapes of the methane and ammonia molecules.
Using Models Why is carbon in the center of the methane molecule?
Writing Formulas for Ionic Compounds
If you know the name of an ionic compound, you can write its formula. Place the symbol of the cation first, followed by the symbol of the anion. Use subscripts to show the ratio of the ions in the compound. Because all compounds are neutral, the total charges on the cations and anions must add up to zero.
Suppose an atom that gains two electrons, such as sulfur, reacts with an atom that loses one electron, such as sodium. There must be two sodium ions (Na+) for each sulfide ion (S2−). The formula for sodium sulfide is Na2S. The 2 − charge on one sulfide ion is balanced by the 1+ charges on two sodium ions.
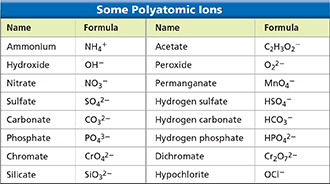
Figure 19 This table lists the names and formulas of some polyatomic ions. Except for the ammonium ion, all the ions listed are anions. Using Tables Which element is found in all the anions whose names end in -ate?
 dd
dd



