Solids
Think about these familiar objects: a pencil, a quarter, a book, and a cafeteria tray. What do these four objects have in common? They all have a recognizable shape and they all take up a certain amount of space. The materials in these objects are all in the solid state. Solid is the state of matter in which materials have a definite shape and a definite volume.
The term definite means that the shape and volume of a pencil won't change as you move the pencil from a desk drawer to a pencil case to a backpack. Changing the container doesn't change the shape or volume of a solid. However, the term definite doesn't mean that the shape or volume can never change. After all, you can change the shape of a pencil by sharpening it. You can change the shape of a copper wire by bending the wire.
Figure 2 shows the arrangement of atoms in a copper wire. The copper atoms are packed close together and are arranged in a regular pattern. Almost all solids have some type of orderly arrangement of particles at the atomic level.
Liquids
How good are you at estimating whether the juice remaining in an almost-empty bottle will fit in a glass? If your estimate is not accurate, you will run out of space in the glass before you run out of juice in the bottle.
Appearances can be deceiving. Imagine a narrow glass and a wide bottle side by side. Each contains exactly 350 milliliters of juice (about three quarters of a pint). There will seem to be more juice in the glass because the juice rises almost to the rim of the glass. There will seem to be less juice in the bottle because the juice forms a shallow layer. What can you learn about liquids from this comparison?
A liquid always has the same shape as its container and can be poured from one container to another. Liquid is the state of matter in which a material has a definite volume but not a definite shape.
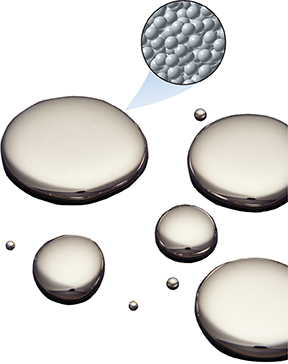
Mercury exists as a liquid at room temperature. The drawing in Figure 3 shows the arrangement of atoms in liquid mercury. Compare this arrangement to the arrangement of copper atoms in Figure 2. The mercury atoms are close together but their arrangement is more random than the arrangement of atoms in copper.
Figure 2 Samples of solid copper have definite volume. Copper atoms are packed close together in an orderly arrangement.
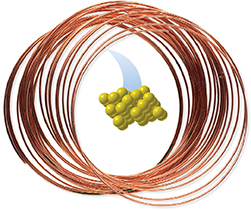
Figure 3 At room temperature, mercury is a liquid. Drops of mercury on a flat, clean surface have a round shape. Mercury in a container has the same shape as its container. Comparing and Contrasting Compare the arrangement of atoms in copper and mercury.