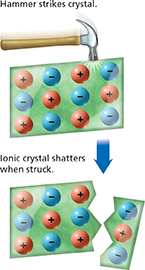
Figure 7 When an ionic crystal is struck, ions are moved from their fixed positions. Ions with the same charge repel one another and the crystal shatters.
Properties of Ionic Compounds
The properties of sodium chloride are typical of an ionic compound. It has a high melting point (801°C). In its solid state, sodium chloride is a poor conductor of electric current. But when melted, it is a good conductor of electric current. Sodium chloride crystals shatter when struck with a hammer.  The properties of an ionic compound can be explained by the strong attractions among ions within a crystal lattice.
The properties of an ionic compound can be explained by the strong attractions among ions within a crystal lattice.
Recall that the arrangement of particles in a substance is the result of two opposing factors. The first factor is the attractions among particles in the substance. The second factor is the kinetic energy of the particles. The stronger the attractions among the particles, the more kinetic energy the particles must have before they can separate.
For an electric current to flow, charged particles must be able to move from one location to another. The ions in a solid crystal lattice have fixed positions. However, when the solid melts, the lattice breaks apart and the ions are free to flow. Melted, or molten, sodium chloride is an excellent conductor of electric current.
Rock salt contains large crystals of sodium chloride. If you tapped a crystal of rock salt sharply with a hammer, it would shatter into many smaller crystals. Figure 7 shows what happens to the positions of the ions when the crystal is struck. Negative ions are pushed into positions near negative ions, and positive ions are pushed into positions near positive ions. Ions with the same charge repel one another and cause the crystal to shatter.
Section 6.1 Assessment
Reviewing Concepts
 When is an atom least likely to react?
When is an atom least likely to react? Describe one way an element can achieve a stable electron configuration.
Describe one way an element can achieve a stable electron configuration. What characteristic of ionic bonds can be used to explain the properties of ionic compounds?
What characteristic of ionic bonds can be used to explain the properties of ionic compounds?Use ionization energy to explain why metals lose electrons more easily than nonmetals.
Why is a rock salt crystal likely to shatter when struck?
Critical Thinking
Making Generalizations What will the ratio of ions be in any compound formed from a Group 1A metal and a Group 7A nonmetal? Explain your answer.
Drawing Conclusions Why do ionic compounds include at least one metal?
Predicting Based on their chemical formulas, which of these compounds is not likely to be an ionic compound: KBr, SO2, or FeCl3? Explain your answer.
Connecting Concepts
Reactivity of Metals Use what you know about how ionic bonds form to explain the difference in reactivity between potassium and calcium. If necessary, reread the description of Group 1A and Group 2A properties in Section 5.3.




