Exploration Lab
Preparing a Salt by Neutralization
In this lab, you will prepare table salt by reacting hydrochloric acid (HCl) with sodium hydroxide (NaOH). To be sure that all of the acid and base have reacted, you will use phenolphthalein. You will first have to test the colors of this indicator with a known acid and base. After the acid and base have reacted, you will measure the pH of the solution with pH paper. Finally, you will evaporate the water and collect the sodium chloride.
Problem How can you produce a salt by neutralization?
Materials
3 dropper pipets
labels
10-mL graduated cylinder
test tube rack
2 10-mL test tubes
distilled water
hydrochloric acid
sodium hydroxide solution
3 stirring rods
phenolphthalein solution
2 25-mL beakers
pH paper
large watch glass
100-mL beaker
hot plate
 For the probeware version of this lab, see Probeware Lab Manual, Lab 3.
For the probeware version of this lab, see Probeware Lab Manual, Lab 3.
Skills Observing, Measuring, Analyzing Data
Procedure 
Part A: Preparing for the Experiment
On a separate sheet of paper, copy the data table shown.
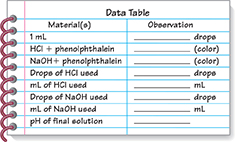
Place about 10 mL of distilled water in a 25-mL beaker. Set the graduated cylinder on the table and add distilled water to the 5-mL mark. Be sure that the bottom of the meniscus is on the 5-mL line.
To determine the number of drops in 1 mL, use a clean dropper pipet to add 1 mL of water to the graduated cylinder. Hold the dropper pipet straight up and down with the tip of the dropper pipet just inside the mouth of the cylinder. As your partner watches the liquid level in the cylinder, add drops of water one at a time while counting the drops. Continue adding drops until the liquid level reaches 6 mL. Record the number of drops in 1 mL.
Label one clean dropper pipet Hydrochloric acid (HCl) and the other Sodium hydroxide (NaOH).
Using the HCl dropper pipet, add 3 mL of hydrochloric acid to a clean test tube. CAUTION Hydrochloric acid is corrosive. In case of spills, clean thoroughly with water. Add 2 to 3 drops of phenolphthalein to the test tube. Use a clean stirring rod to mix the hydrochloric acid and indicator. Record your observations.
Using the dropper pipet labeled NaOH, add 3 mL of sodium hydroxide solution to a clean test tube. CAUTION Sodium hydroxide is corrosive. In case of spills, clean thoroughly with water. Add 2 to 3 drops of phenolphthalein to the test tube. Use a clean stirring rod to mix the sodium hydroxide solution and indicator. Record your observations.
Part B: Making the Salt
Using the HCl dropper pipet, add 4 mL of hydrochloric acid to a clean 25-mL beaker. Record the number of drops you used. Add 2 to 3 drops of phenolphthalein to the beaker.
Use the NaOH dropper pipet to add sodium hydroxide drop by drop to the beaker of hydrochloric acid and phenolphthalein, stirring constantly. Count the drops as you add them. As a pink color remains longer, add the drops more slowly.
End ofPage 254




