Figure 8 The petroleum pumped from underground deposits is a complex mixture of hydrocarbons. A At a refinery, petroleum is separated into mixtures called fractions. B The diagram shows some of the fractions that can be collected from a distillation tower. The labels provide data on the compounds within a fraction. For example, compounds in kerosene have from 12 to 16 carbon atoms and condense at temperatures between 200°C and 250°C. Interpreting Diagrams How many carbon atoms are there in the compounds in the petroleum gas fraction?
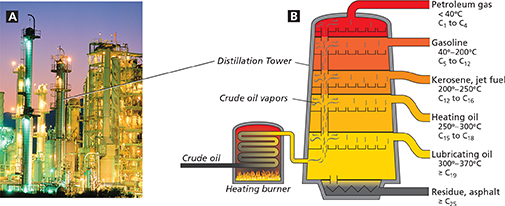
The diagram in Figure 8B shows how petroleum is separated into fractions through a type of distillation called fractional distillation. When petroleum is heated in a distillation tower, most of the hydrocarbons vaporize. The vapors rise through the tower and condense as the temperature decreases. Compounds with higher boiling points condense first. They are collected near the bottom of the tower.
Combustion of Fossil Fuels
The energy released from fossil fuels through combustion is used to heat buildings, cook food, or for transportation. Recall that energy from the combustion of propane heats the air in a hot-air balloon.
 The primary products of the complete combustion of fossil fuels are carbon dioxide and water. Burning fossil fuels increases the amount of carbon dioxide in the atmosphere. This increase may affect temperatures, amounts of rain, and sea levels worldwide. Some sulfur and nitrogen are in fossil fuels, and air contains nitrogen. So nitrogen oxides and sulfur dioxide are produced during the combustion of fossil fuels.
The primary products of the complete combustion of fossil fuels are carbon dioxide and water. Burning fossil fuels increases the amount of carbon dioxide in the atmosphere. This increase may affect temperatures, amounts of rain, and sea levels worldwide. Some sulfur and nitrogen are in fossil fuels, and air contains nitrogen. So nitrogen oxides and sulfur dioxide are produced during the combustion of fossil fuels.
Incomplete Combustion
In stoves and furnaces, there may not be enough oxygen available for complete combustion of all the fuel. So a deadly gas, carbon monoxide, is produced. This colorless, odorless gas can be inhaled and absorbed by blood. It keeps hemoglobin from carrying oxygen to cells. Safety experts recommend that homes heated with natural gas or heating oil have carbon monoxide detectors.
When fossil fuels undergo incomplete combustion in factories or power plants, they also produce tiny particles of carbon. Inhaling these particles can cause heart and lung problems.





