During gamma decay, the atomic number and mass number of the atom remain the same, but the energy of the nucleus decreases. Gamma decay often accompanies alpha or beta decay. For example, thorium-234 emits both beta particles and gamma rays (abbreviated as γ) as it decays.
Gamma rays are much more penetrating than either alpha particles or beta particles. It can take several centimeters of lead or several meters of concrete to stop gamma radiation.
Figure 5 The mineral autunite is an important source of uranium.

Effects of Nuclear Radiation
You may not realize it, but you are exposed to nuclear radiation every day. Most of this is background radiation, or nuclear radiation that occurs naturally in the environment. Radioisotopes in air, water, rocks, plants, and animals all contribute to background radiation. Most rocks, such as the one in Figure 5, contain at least trace amounts of radioactive elements. Another source of background radiation is cosmic rays. Cosmic rays are streams of charged particles (mainly protons and alpha particles) from outer space. Collisions between cosmic rays and Earth's atmosphere shower the surface below with nuclear radiation. All this radioactivity may sound dangerous. However, background radiation levels are generally low enough to be safe.
When nuclear radiation exceeds background levels, it can damage the cells and tissues of your body.  Nuclear radiation can ionize atoms. When cells are exposed to nuclear radiation, the bonds holding together proteins and DNA molecules may break. As these molecules change, the cells may no longer function properly.
Nuclear radiation can ionize atoms. When cells are exposed to nuclear radiation, the bonds holding together proteins and DNA molecules may break. As these molecules change, the cells may no longer function properly.
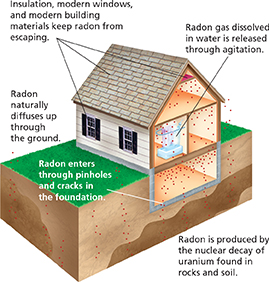
Alpha particles, beta particles, and gamma rays are all forms of ionizing radiation. Alpha particles can cause skin damage similar to a burn, but they are not a serious health hazard unless an alpha-emitting substance is inhaled or eaten. For example, radon gas is a potentially dangerous natural source of alpha particles because it can be inhaled. Radon-222 is formed through a series of nuclear decays that begins with uranium-238 in rocks deep underground. As radon-222 is produced, it seeps upward toward the surface. It sometimes collects in the basements of buildings that lack proper ventilation, as shown in Figure 6. Prolonged exposure to radon-222 can lead to lung cancer.
Figure 6 Radon gas is produced underground as the uranium in rocks and soil decays. As the radon seeps up through the ground, it can get into buildings by passing through cracks or holes in their foundations.
Inferring How would ventilation of the basement affect radon levels in the house shown below?
 d
d



