Standardized Test Prep
Test-Taking Tip
Evaluating and Revising
Frequently, a scientifically accurate answer choice may not answer the question that is being asked. Keep these tips in mind:
Verify what the question is asking.
Determine if an answer choice is a true statement or not.
Determine if a true answer choice actually answers the question.
Be cautious with inserted or deleted words that make a false statement seem accurate.
Practice using these tips in Question 5.
Choose the letter that best answers the question or completes the statement.
Which equation correctly shows beta decay?
The half-life of radon-222 is 3.8 days. If a sample currently has 3.1 grams of radon-222, how much radon-222 did this sample have 15.2 days ago?
12.4 grams
47.1 grams
49.6 grams
57.8 grams
92.7 grams
Radioactive decay of nuclei often involves several decays before a stable nucleus is formed. This is called a decay chain. What stable isotope is formed when radon-222 undergoes a decay chain of four alpha decays followed by four beta decays?
tungsten-206
platinum-206
lead-206
tungsten-214
lead-214
Which nucleus balances the following nuclear equation for the fission of uranium-235?
Uranium-238 is less stable than oxygen-16. What accounts for this difference?
Uranium is a solid, while oxygen is a gas.
Unlike oxygen-16, uranium-238 has a nucleus in which repulsive electric forces surpass the strong nuclear forces.
Oxygen-16 has fewer electrons than uranium-238.
Uranium-238 has fewer neutrons than oxygen-16.
Unlike uranium-238, oxygen-16 has a nucleus in which the strong nuclear forces are overcome by repulsive electric forces.
The primary source of energy in stars is the fusion of hydrogen into helium. However, another reaction is believed to occur simultaneously. It is called the carbon-nitrogen-oxygen (CNO) cycle. In the diagram below, the symbol
 represents a positron. A positron is a particle that has the same mass as an electron but a charge of 1+.
represents a positron. A positron is a particle that has the same mass as an electron but a charge of 1+.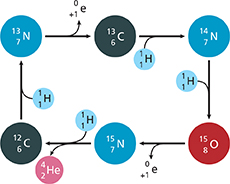
Which equation describes the CNO cycle?





