Volume
Imagine that you have a plastic bottle that appears empty. If you twist the cap onto the bottle and then squeeze the bottle, what will happen? At first, the plastic will give a little, reducing the volume of the bottle. But soon you will feel pressure from inside the bottle resisting your efforts to further reduce the volume. The pressure you feel is a result of the increased pressure of the air trapped inside the bottle. As the volume is decreased, particles of trapped air collide more often with the walls of the bottle.  Reducing the volume of a gas increases its pressure if the temperature of the gas and the number of particles are constant.
Reducing the volume of a gas increases its pressure if the temperature of the gas and the number of particles are constant.
Figure 12 shows how the relationship between volume and pressure explains what happens when you breathe. As you inhale, a muscle called the diaphragm (DY uh fram) contracts. The contraction causes your chest cavity to expand. This temporary increase in volume allows the particles in air to spread out, which lowers the pressure inside the chest cavity. Because the pressure of the air outside your body is now greater than the pressure inside your chest, air rushes into your lungs.
Figure 12 Movement of a muscle called the diaphragm changes the volume of your chest cavity. The volume increases when you inhale and decreases when you exhale.
Interpreting Diagrams How does the movement of your rib cage affect the volume of your chest cavity?
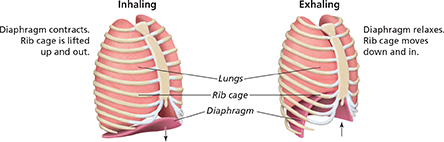
When you exhale, your diaphragm relaxes and the volume of your chest cavity decreases. The particles in the air are squeezed into a smaller volume and the pressure inside your lungs increases. Because the pressure of the air inside your chest is now greater than the pressure of the air outside your body, air is forced out of your lungs.
Number of Particles
You can probably predict what will happen to the pressure when you add more gas to a container. Think about a tire. Once the tire is inflated, its volume is fairly constant. So adding more air will increase the pressure inside the tire. The more particles there are in the same volume, the greater the number of collisions and the greater the pressure. At some point the rubber from which the tire is made will not be strong enough to withstand the increased pressure and the tire will burst.  Increasing the number of particles will increase the pressure of a gas if the temperature and the volume are constant.
Increasing the number of particles will increase the pressure of a gas if the temperature and the volume are constant.





