Critical Thinking
Classifying If you take a helium balloon from inside a warm house to outside on a snowy day, what will happen to the balloon? Could you classify this change as a phase change? Explain your answer.
Comparing and Contrasting Compare the melting and freezing of water in terms of (a) the temperature at which these processes take place and (b) how energy is involved in these processes.
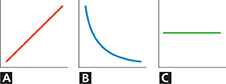
Use the graphs to answer Questions 27—29.
Using Graphs Which graph represents what happens to the pressure in a tire as air is added to the tire? Assume the temperature of the gas is constant.
Using Graphs Which graph represents what happens to the pressure in an aerosol can if the can is heated?
Applying Concepts Which graph represents temperature versus time during a phase change?
Math Skills
Making Generalizations The pressure of a gas is directly proportional to its temperature in kelvins. Using P1, P2, T1, and T2, write a mathematical equation that expresses this relationship.
Calculating An automobile tire has a pressure of 325 kPa when the temperature is 10°C. If the temperature of the tire rises to 50°C and its volume is constant, what is the new pressure?
Calculating A gas sample occupies 4.2 L at a pressure of 101 kPa. What volume will it occupy if the pressure is increased to 235 kPa?
Concepts in Action
Using Models If there is a gas leak in the basement of a building, you will soon notice an odor throughout the house. However, if there is a water leak in the basement, you will need to go to the basement to detect the leak. Use the kinetic theory to explain the differences.
Inferring A student examines a thermometer placed in a can containing a substance that is being heated. The temperature remains the same for several minutes, and then it starts to rise. Without looking in the can, how does the student know what is occurring in the can?
Relating Cause and Effect In Earth's atmosphere, pressure and temperature both decrease as altitude increases. Weather balloons expand as they rise. Which has more effect on the weather balloon, the decrease in pressure or the decrease in temperature? Explain your answer.
Drawing Conclusions In a car engine, air and gasoline vapors are mixed in a cylinder. A piston is pushed into the cylinder before a spark ignites the mixture of gases. When the piston is pushed into the cylinder, what happens to the pressure of the gases in the cylinder?
Writing in Science Unpopped popcorn kernels contain a small amount of water. Use what you know about vaporization and how gases behave to explain why popcorn pops when it is heated.
Performance-Based Assessment
Making a Poster The gas laws have many practical applications in cooking. Make a poster, including diagrams, that shows how two factors that affect gases affect cooking. Examples might include explaining why cakes rise while baking or why some recipes specify high-altitude temperatures and cooking times.