The Noble Gases
The elements in Group 8A are called noble gases. Helium has two valence electrons. Each of the other noble gases has eight valence electrons.  The noble gases are colorless and odorless and extremely unreactive. In Chapter 6, you will study the relationship between the electron configurations of the noble gases and their low reactivity.
The noble gases are colorless and odorless and extremely unreactive. In Chapter 6, you will study the relationship between the electron configurations of the noble gases and their low reactivity.
It is not easy to discover a colorless, odorless gas. It is even harder if the gas rarely reacts. Scientists discovered argon when they noticed that the density of nitrogen collected from air did not match the density of nitrogen formed during chemical changes. In time, the scientists figured out that the “impurity” in atmospheric nitrogen was an unknown element.
An element that does not react easily with other elements can be very useful. For example, during one stage in the process of making computer chips, pure silicon is heated in a furnace at 1480°C. At this temperature, silicon reacts with both oxygen and nitrogen. So the heating must take place in an argon atmosphere.
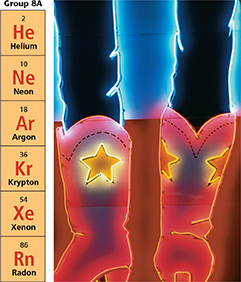
Some light bulbs are filled with argon because the glowing filament in the bulb will not react with argon as it would react with oxygen. Using argon increases the number of hours the bulb can be lit before it burns out. All the noble gases except radon are used in “neon” lights like those shown in Figure 22.
Figure 22 When electric current passes through noble gases, they emit different colors. Helium emits pink, neon emits orange-red, argon emits lavender, krypton emits white, and xenon emits blue.
Section 5.3 Assessment
Reviewing Concepts
 Explain why elements in a group have similar properties.
Explain why elements in a group have similar properties. What is the relationship between an alkali metal's location in Group 1A and its reactivity?
What is the relationship between an alkali metal's location in Group 1A and its reactivity? What element exists in almost every compound in your body?
What element exists in almost every compound in your body? Which Group 5A elements are found in fertilizer?
Which Group 5A elements are found in fertilizer? Which group of elements is the least reactive?
Which group of elements is the least reactive?Why is hydrogen located in a group with reactive metals?
What biological function requires magnesium?
Why is aluminum recycled?
What is the main use of sulfur?
Why is chlorine added to drinking water?
Critical Thinking
Comparing and Contrasting In which class of elements is there a greater range of properties, the metals or the nonmetals? Give an example to support your answer.
Making Generalizations What happens to the reactivity of nonmetals within a group from the top of the group to the bottom?




