Critical Thinking
Observing Explain how you know that a chemical reaction takes place when iron rusts.
Inferring As a candle burns, its mass decreases. However, mass is conserved in this reaction. Explain this observation.
Making Generalizations In a certain chemical reaction, two reactants undergo change to form two products. Why can't you determine what type of reaction occurred from this information?
Applying Concepts Use the reaction that occurs when magnesium burns in oxygen to show how a reaction might be included in more than one category of reaction.
Classifying Is breaking bonds an endothermic process or an exothermic process? Explain.
Inferring Explain how energy is conserved in an endothermic reaction.
Use the diagram below to answer Questions 25–26.
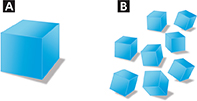
Comparing and Contrasting The volume of the cube in A equals the total volume of the cubes in B. Compare the surface areas of the cubes in A and B.
Using Models Use the diagram to explain how surface area affects reaction rates.
Predicting You are performing an experiment and want to increase the rate of reaction. You stop stirring the reactants and, instead, increase the concentration of one reactant. Can you expect the reaction to proceed at a faster rate? Explain.
Math Skills
Applying Concepts Balance each of the following chemical equations.
Calculating What mass of KBr is contained in 2.50 moles of the compound?
Calculating How many moles of Na2CrO4 are contained in 74.3 grams of the compound?
Concepts in Action
Inferring Kept at room temperature, batteries will eventually lose their charge. Why will keeping batteries in the freezer make them last longer?
Drawing Conclusions Octane, C8H18, is one of the compounds present in gasoline. The products of the burning of octane are water and carbon dioxide. In this reaction, which contain more energy, the bonds in the reactants or the bonds in the products? Explain.
Writing in Science Write a paragraph describing a chemical reaction that you have observed recently.
Performance-Based Assessment
Designing an Experiment Choose a chemical reaction that reaches equilibrium and includes at least one gas. Design an experiment that will show the effects of concentration, temperature, and pressure on the system. You will not actually perform the experiment, so you are not limited by the equipment available in your laboratory. Prepare a lab report that includes a hypothesis, all steps of the procedure, and any expected results.





