Factors Affecting Solubility
Have you ever had to clean oil or grease from your hands? If you rinse your hands in water alone, the oil remains on your hands. But if you use soapy water, you can easily rinse the oil off your hands. Oil is soluble in soapy water, but not in pure water. Solubility varies not only with the solvent used, but also with the conditions of the solution process.  Three factors that affect the solubility of a solute are the polarity of the solvent, temperature, and pressure.
Three factors that affect the solubility of a solute are the polarity of the solvent, temperature, and pressure.
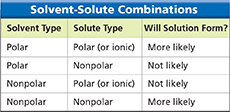
Figure 10 Generally, a solute is more likely to dissolve in a “like” solvent than an “unlike” solvent. Classifying How is saltwater an example of “like dissolving in like”?
 d
dPolar and Nonpolar Solvents
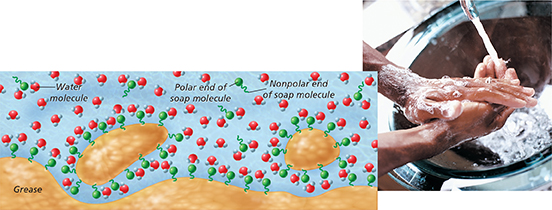
Oil does not dissolve in water because oil molecules are nonpolar and water molecules are polar. A common guideline for predicting solubility is “like dissolves like.” Solution formation is more likely to happen when the solute and solvent are either both polar or both nonpolar. Figure 11 illustrates how soapy water dissolves oil. A soap molecule has a polar end, which attracts water molecules, and a nonpolar end, which attracts oil. The soap molecules break up the oil into small droplets that are soluble in water.
Temperature
When you add a large amount of sugar to cold tea, only a small amount dissolves. If you add the same amount of sugar to the same amount of hot tea, more sugar will dissolve. In general, the solubility of solids increases as the solvent temperature increases.
When a glass of cold water warms up to room temperature, bubbles form on the inside of the glass. These bubbles are gases that were dissolved in the water. They come out of the solution as the water temperature rises. Unlike most solids, gases usually become less soluble as the temperature of the solvent increases.
Pressure
How do manufacturers produce a carbonated beverage? They use pressure to force carbon dioxide (CO2) to dissolve in the liquid. Increasing the pressure on a gas increases its solubility in a liquid. The pressure of CO2 in a sealed 12-ounce can of soda at room temperature can be two to three times atmospheric pressure.
Figure 11 Soaps and detergents are used to remove grease and oil stains. Soap molecules form attractions to both polar water molecules and nonpolar oil molecules. As the water flows away, it carries the oil with it.