Figure 14 Synthetic polymers are used to make tires, ropes, and plastic objects. A About half the rubber produced in the world is used to manufacture tires. B Nylon is a good choice for a rope because its fibers are strong and do not wear out easily. C The hard plastic shapes are made from a high-density polyethylene polymer. Inferring At room temperature, polymers are most likely to exist as which state of matter?

Synthetic Polymers
The properties of a polymer depend on the type and number of monomers in the polymer.  Rubber, nylon, and polyethylene are three examples of compounds that can be synthesized.
Rubber, nylon, and polyethylene are three examples of compounds that can be synthesized.
Rubber
The sap collected from rubber trees in tropical regions contains rubber. So why would a chemist make synthetic rubber? The supply of natural rubber is limited. During World War II, the allies could not obtain natural rubber. Chemists worked hard to produce a synthetic rubber, using hydrocarbons from petroleum. Natural rubber and synthetic rubbers contain different monomers and have different properties. The tires in Figure 14A will resist wear and be less likely to leak if they are made of synthetic rubber. Rubber is used as an adhesive. The How It Works box on page 277 explains how adhesives work.
Nylon
In the 1930s, Wallace Carothers was trying to produce a synthetic polymer to replace silk. The polymer he produced was nylon, which has properties not found in natural polymers. Nylon fibers are very strong, durable, and shiny. Nylon is used in parachutes, wind-breakers, fishing line, carpets, and ropes like the one in Figure 14B.
Polyethylene
Plastic milk bottles, plastic wrap, and the plastic shapes in Figure 14C are made of polyethylene. This polymer forms when ethene (or ethylene) molecules link head to tail. The number of carbon atoms in a polyethylene chain affects the properties of the polymer. The more carbon atoms in the chain, the harder the polymer is.
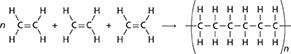

What determines the hardness of polyethylene?





